Abstract
The cerebrovascular response to decreases in hematocrit and viscosity depends on accompanying changes in arterial O2 content. This study examines whether 1) the arteriolar dilation seen after exchange transfusion with a 5% albumin solution can be reduced by the KATP channel antagonist glibenclamide (known to inhibit hypoxic dilation), and 2) the arteriolar constriction seen after exchange transfusion with a cell-free hemoglobin polymer to improve O2-carrying capacity can be blocked by inhibitors of the synthesis or vasoconstrictor actions of 20-HETE. In anesthetized rats, decreasing hematocrit by one-third with albumin exchange transfusion dilated pial arterioles (14 ± 2%; SD), whereas superfusion of the surface of the brain with 10 μM glibenclamide blocked this response (−10 ± 7%). Exchange transfusion with polymeric hemoglobin decreased the diameter of pial arterioles by 20 ± 3% without altering arterial pressure. This constrictor response was attenuated by superfusing the surface of the brain with a 20-HETE antagonist, WIT-002 (10 μM; −5 ± 1%), and was blocked by two chemically dissimilar selective inhibitors of the synthesis of 20-HETE, DDMS (50 μM; 0 ± 4%) and HET-0016 (1 μM; −6 ± 4%). The constrictor response to hemoglobin transfusion was not blocked by an inhibitor of nitric oxide (NO) synthase, and the inhibition of the constrictor response by DDMS was not altered by coadministration of the NO synthase inhibitor. We conclude 1) that activation of KATP channels contributes to pial arteriolar dilation during anemia, whereas 2) constriction to polymeric hemoglobin transfusion at reduced hematocrit represents a regulatory response that limits increased O2 transport and that is mediated by increased formation of 20-HETE, rather than by NO scavenging.
Keywords: anemia, blood substitute, cytochrome P-450, nitric oxide, oxygen carrier, ATP-sensitive potassium channel
Cerebrovascular Autoregulation usually refers to active changes in arteriolar diameter that maintain cerebral blood flow constant during alterations in cerebral perfusion pressure. With increases in arterial blood pressure, the normal maintenance of cerebral blood flow has been shown to depend, in part, on cytochrome P-450 (CYP) ω-hydroxylase activity, which converts arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE) in arterial smooth muscle (5). 20-HETE is thought to amplify the myogenic response by inhibiting calcium-activated potassium channels (6, 16), which would ordinarily temper an increase in tone elicited by the increases in intracellular calcium. Production of 20-HETE is O2 dependent in the physiological range (7). Thus interventions that increase oxygenation in the brain other than an increase in perfusion pressure may also act to produce vasoconstriction by a mechanism dependent on increased formation of 20-HETE.
Changes in pial arteriolar diameter in response to changes in blood viscosity have been referred to as viscosity autoregulation (21, 23). Decreases in arterial hematocrit have been reported to either increase, decrease, or produce no change in pial arteriolar diameter (4, 11, 12, 22, 23, 25). This variability may be due to the countering effects of viscosity and changes in arterial O2 content on oxygenation and, thus, may be influenced by differences in baseline hematocrit and metabolic rate for a particular species. We previously reported that exchange transfusion of a solution of cell-free tetrameric cross-linked hemoglobin or cell-free polymeric hemoglobin in cats constricts pial arterioles, in contrast to the dilation seen with exchange transfusion of an albumin solution at equivalent hematocrit but with a lower O2-carrying capacity (4, 25). Interestingly, the different arteriolar responses to transfusion with albumin- vs. hemoglobin-containing solutions produced equivalent levels of cerebral O2 transport, but increasing plasma viscosity during the cell-free hemoglobin transfusion converted the constrictor response to a dilator response. These findings imply that active vascular responses to changes in viscosity of the blood can be viewed as an extension of pressure-induced autoregulation in which Po2 levels in the brain are tightly controlled.
Without active arteriolar constriction, decreases in blood viscosity without a fall in arterial O2 content will increase in cerebral blood flow and increase tissue Po2. We postulate that the pial arteriolar constrictor response seen after exchange transfusion with cell-free, polymeric hemoglobin solution may be mediated by an O2-dependent increase in ω-hydroxylase activity. The possibility that changes in O2 levels in the brain contributes to viscosity autoregulation by regulating the production of the potent vasoconstrictor 20-HETE in cerebral arteries has not been investigated previously. In addition, ω-hydroxylase activity is strongly inhibited by nitric oxide (NO) (1, 30). Because it has been postulated that cell-free hemoglobin in the plasma can act as a more efficient sink for NO than cell-based hemoglobin (10, 17, 18), an alternative possibility is that plasma-based hemoglobin reduces NO concentration in vascular smooth muscle sufficiently to disinhibit ω-hydroxylase activity. Thus the present study examined the hypotheses 1) that local application of a 20-HETE antagonist or two chemically dissimilar selective inhibitors of the synthesis of 20-HETE (2, 15, 20) reduces the pial arteriolar constrictor response to exchange transfusion of a cell-free polymeric hemoglobin solution, 2) that local application of a NO synthase (NOS) inhibitor does not block the pial arteriolar constrictor response to polymeric hemoglobin transfusion and does not interfere with the inhibitory effect of an ω-hydroxylase inhibitor on the response, and 3) that the pial arteriolar dilation seen after exchange transfusion with an albumin-containing solution is reduced by local application of an inhibitor of ATP-sensitive potassium (KATP) channels. The rationale for the latter hypothesis is based on the assumption that the dilation is the result of a hypoxic stimulus accompanying the decrease in O2-carrying capacity (34, 36) and on the evidence that KATP channels contribute to pial arteriolar dilation associated with hypoxic hypoxia (29, 31).
METHODS
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Male Wistar rats weighing 250–300 g were anesthetized with halothane + 70% N2O-balance O2. The lungs were mechanically ventilated through a tracheostomy. Catheters were inserted into a femoral artery and vein for monitoring arterial blood pressure and for infusions. A closed cranial window was constructed over the parietal cortex for measuring pial arteriolar diameter by intravital microscopy (3, 9). A plastic ring was cemented to the skull, and ~3–4 mm of bone were removed. The dura was incised and retracted, the ring was filled with artificial cerebrospinal fluid (CSF), and the window was sealed with a coverslip cemented to the plastic ring. The plastic ring was equipped with an inflow port, an outflow port, a port for measuring fluid pressure, and a thermistor for monitoring fluid temperature. Rectal temperature was maintained at ~37°C with a heating pad.
Pial arterioles were imaged through the cranial window with a microscope and video recording system. Measurements were usually made at two to four sites along each arteriole segment and on two different arterioles in each window. For each intervention, the percent change in arteriolar diameter was calculated at each site. An average percent change was then obtained for each rat, and this average value was used for statistical analysis, with the sample size equal to the number of rats.
Exchange transfusion was performed over a 15-min period by administration of a 5% solution of human albumin or a 6% solution of a hemoglobin polymer. Approximately 7 ml of blood were removed from the rat via the arterial catheter while simultaneously being replaced with an intravenous infusion of either solution to reduce hematocrit by ~30%. The process for polymerization, purification, and removal of endotoxin from the solution of the bovine hemoglobin polymer in lactated Ringer solution has been previously described (19, 25). The polymers are characterized by covalent amide bonds linking the tetramers (zero-link bovine hemoglobin polymer). Low-molecular-mass species (<300 kDa) were removed from the hemoglobin solution by diafiltration in which the retentate contains polymers with an estimated average molecular mass of 20 MDa. The polymeric hemoglobin has a high O2 affinity (P50 ~4 Torr at 50% O2 saturation) with poor cooperativity (Hill coefficient of ~1).
To assess the role of 20-HETE in the arteriolar response to hemoglobin transfusion, the window was superfused with either 1) the putative 20-HETE antagonist WIT-002 [20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (also known as 20-HEDE); 10 μM] (5, 37); 2) a selective inhibitor of the synthesis of 20-HETE, DDMS (N-methylsulfonyl-12,12-dibromododec-11-enamide; 50 μM) (5, 35); or 3) a structurally dissimilar inhibitor of the synthesis of 20-HETE, HET-0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine; 1 μM] (15, 20). The concentrations of these inhibitors have previously been shown to be efficacious in the cerebral circulation of rats (5, 15). To test the role of NO in the vascular response to hemoglobin transfusion, the window was superfused with the NOS inhibitor Nω-nitro-L-arginine (L-NNA; 300 μM). To test the role of KATP channels in the arteriolar response to albumin exchange transfusion, the window was superfused with 10 μM glibenclamide. Vehicle or a single drug diluted in artificial CSF was superfused through the cranial window at a rate of 0.2 ml/min for 20 min before the start of the exchange transfusion. Fluid pressure in the window was maintained at 5 mmHg by adjusting the height of the outflow catheter.
Six groups of rats were studied with hemoglobin exchange transfusion: 1) vehicle (0.25% ethanol; n = 6); 2) WIT-002 (n = 6); 3) DDMS (n = 7); 4) HET-0016 (n = 6); 5) L-NNA (n = 6); and 6) L-NNA + DDMS (n = 6). The combined L-NNA + DDMS group was studied to determine whether the effect of DDMS on the vascular response required NOS activity. Two groups of rats were studied with albumin transfusion: 1) vehicle (0.1% DMSO; n = 6); and 2) glibenclamide (n = 6).
The percent change in arteriolar diameter was measured immediately after completion of the exchange transfusion and 30 and 60 min later. To test whether the pial arterioles were still capable of constriction after the various drug treatments, the vascular response to superfusion of the pial window with the thromboxane analog U-46619 (0.1 μM) was determined at the end of each experiment.
Statistics
Values are presented as means ± SD. The significance of differences in the percent change in arteriolar diameter at various time points in drug- and vehicle-treated groups was evaluated by analysis of variance and the Newman-Keuls multiple-range test. A P value <0.05 was considered to be significant. Arterial blood measurements made after transfusion were compared with the pretransfusion values by paired t-test.
RESULTS
Exchange transfusion with the zero-link bovine hemoglobin polymer decreased hematocrit from 36–40 to 27–29%. This was accompanied by only a small decrease in blood hemoglobin concentration (Table 1). In contrast, exchange transfusion with the albumin solution produced a large decrease in blood hemoglobin concentration, with a similar reduction in hematocrit. Arterial Pco2 was controlled in the range of 36–40 Torr and was not significantly altered by the transfusion. Arterial Po2 was maintained at ~130 Torr in all rats, and arterial pH was maintained in the range of 7.40–7.45 (data not shown). Mean arterial blood pressure was not significantly changed after transfusion of hemoglobin polymer, although a small decrease occurred after transfusion of the albumin solution (Table 1). Rectal temperature and fluid temperature in the cranial window were both in the range of 36.8–37.5°C before and after transfusion in all groups.
Table 1.
Arterial blood sample analysis and MABP before and after transfusion of solutions of hemoglobin polymer or albumin
| Hematocrit, %
|
Hemoglobin, g/dl
|
Paco2, Torr
|
MABP, mmHg
|
|||||
|---|---|---|---|---|---|---|---|---|
| Pretransfusion | Posttransfusion | Pretransfusion | Posttransfusion | Pretransfusion | Posttransfusion | Pretransfusion | Posttransfusion | |
| Hemoglobin groups | ||||||||
| Vehicle | 36±3 | 27±4* | 11.0±0.7 | 9.7±1.1* | 37±3 | 37±4 | 95±7 | 94±10 |
| WIT-002 | 38±1 | 29±1* | 12.0±0.5 | 10.6±0.2* | 38±2 | 38±2 | 100±4 | 98±4 |
| DDMS | 38±2 | 28±4* | 11.9±0.6 | 9.7±1.7* | 37±1 | 39±3 | 97±13 | 100±12 |
| HET-0016 | 40±2 | 27±3* | 12.5±0.6 | 10.4±0.9* | 39±1 | 39±3 | 94±4 | 97±5 |
| L-NNA | 39±2 | 30±3* | 12.1±0.7 | 10.7±0.8* | 37±2 | 37±2 | 98±8 | 98±9 |
| L-NNA + DDMS | 38±2 | 27±2* | 11.6±0.5 | 10.7±0.4 | 37±1 | 37±1 | 95±9 | 97±9 |
| Albumin groups | ||||||||
| Vehicle | 39±1 | 25±1* | 12.0±0.4 | 7.6±0.3* | 38±2 | 40±3 | 98±5 | 93±6* |
| Glibenclamide | 39±2 | 24±2* | 12.1±1.4 | 7.9±0.7* | 39±2 | 40±2 | 99±12 | 97±10 |
Values are means ± SD. Paco2, arterial Pco2; MABP, mean arterial blood pressure; WIT-002, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid; DDMS, N-methylsulfonyl-12,12-dibromododec-11-enamide; HET-0016, N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine; L-NNA, Nω-nitro-L-arginine.
P < 0.05 from pretransfusion by paired t-test.
Immediately after completion of the exchange transfusion with the polymeric hemoglobin solution, the diameter of the pial arterioles fell by 20%. This constrictor response was attenuated by 75% by superfusing the surface of the brain with 10 μM WIT-002 and was completely blocked in the groups superfused with 50 μM DDMS and 1 μM HET-0016 (Fig. 1).
Fig. 1.
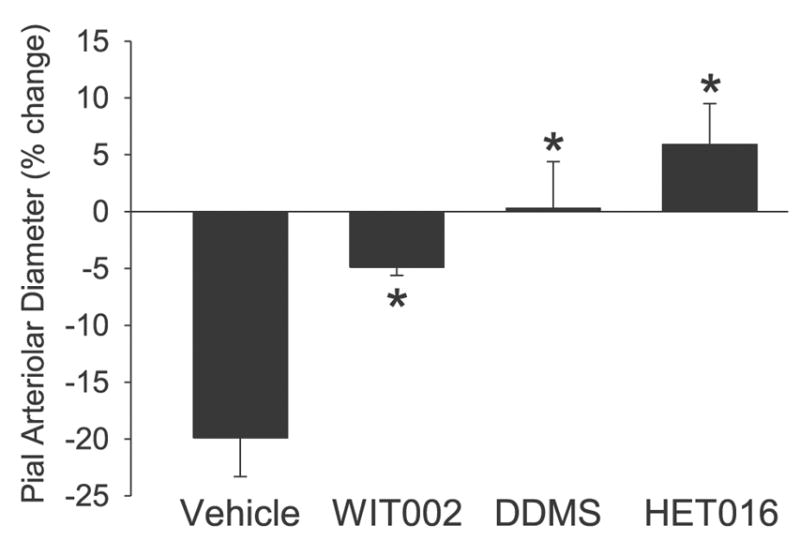
Change in pial arteriolar diameter immediately after completion of an exchange transfusion of the hemoglobin polymer solution expressed as a percentage of the diameter after superfusing the cranial window with 0.25% ethanol vehicle (n = 6), 10 μM 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (WIT-002; n = 6), 50 μM N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS; n = 7), or 1 μM N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET-0016; n = 6). Values are means ± SD. *P < 0.05 from vehicle group.
Previous studies have indicated that the formation of 20-HETE is directly dependent on tissue Po2 levels in the physiological range from 20–100 Torr and is strongly inhibited in cerebral arteries both in vivo and in vitro by NO (1, 7, 30). Because the plasma-based hemoglobin might scavenge NO sufficiently to disinhibit ω-hydroxylase activity, the effect of the NOS inhibitor L-NNA was tested to determine whether L-NNA prevented the ability of DDMS to block the vascular response to hemoglobin transfusion. Superfusion of L-NNA alone reduced the baseline diameter of the pial arterioles by 12% before hemoglobin transfusion (Fig. 2). Subsequent transfusion of hemoglobin produced a statistically significant, additional 5% constriction. The overall constrictor response as a percent of the pre-L-NNA baseline (17%) was similar to that obtained with hemoglobin transfusion in the vehicle-super-fused group. In the vehicle group, the constrictor response gradually subsided over the 60-min observation period after completion of the hemoglobin transfusion (Fig. 2). The time course of the overall constrictor response in the group super-fused with L-NNA was similar to that in the vehicle group over the first 30 min, but the constriction was maintained for more than 60 min after transfusion. In the group superfused with DDMS, time-dependent dilation occurred after hemoglobin transfusion, and the responses were significantly different from those seen in the vehicle group at 0 and 30 min after transfusion. With combined L-NNA and DDMS superfusion, no significant change was observed in baseline diameter before hemoglobin transfusion, in contrast to the constriction seen with L-NNA alone (Fig. 2). Hemoglobin transfusion in the group superfused with L-NNA plus DDMS resulted in time-dependent dilation, which was similar to that seen with DDMS alone. These responses are significantly different from the response seen in rats treated with L-NNA alone at all time points. The difference in the percent diameter responses between 0 min and 60 min after completion of the hemoglobin transfusion was greater in the vehicle group than in any of the drug-treated groups.
Fig. 2.
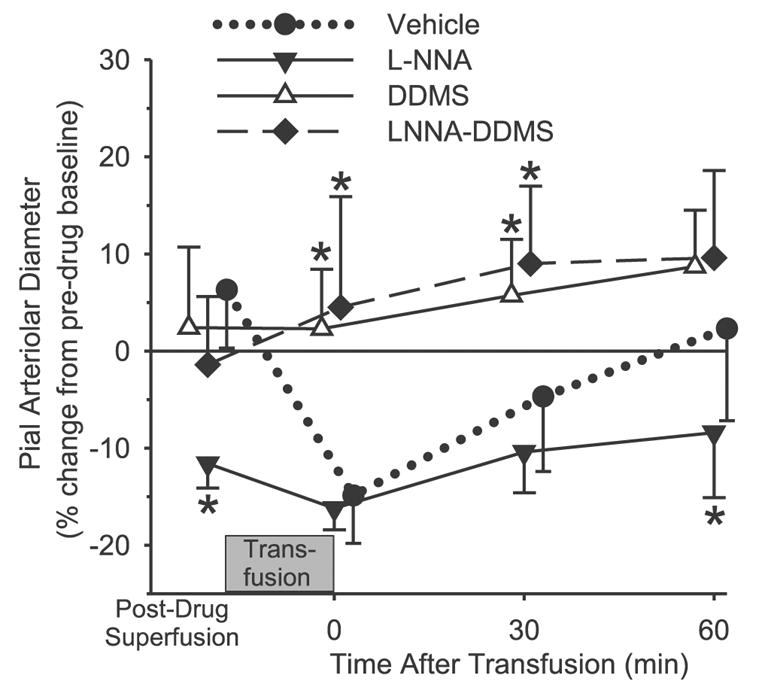
Change in pial arteriolar diameter after superfusion of the cranial window with 0.25% ethanol vehicle (n = 6), 300 μM Nω-nitro-L-arginine (L-NNA; n = 6), 50 μM DDMS (n = 7), or 300 μM L-NNA plus 50 μM DDMS (n = 6), and at 0, 30, and 60 min after completion of an exchange transfusion of the hemoglobin polymer solution. Values are means ± SD and are expressed as a percentage of the baseline diameter before vehicle or drug superfusion of the window. *P < 0.05 from vehicle group.
Exchange transfusion with the albumin solution produced a 14% dilation of pial arteries relative to the baseline diameter after vehicle superfusion. The dilation did not vary over the 60-min observation period (Fig. 3), and the response was significantly different from that seen with hemoglobin transfusion over the entire 60 min. Superfusion of 10 μM glibenclamide did not affect baseline arteriolar diameter compared with vehicle but completely blocked the dilator response to albumin transfusion at all time points (Fig. 3).
Fig. 3.
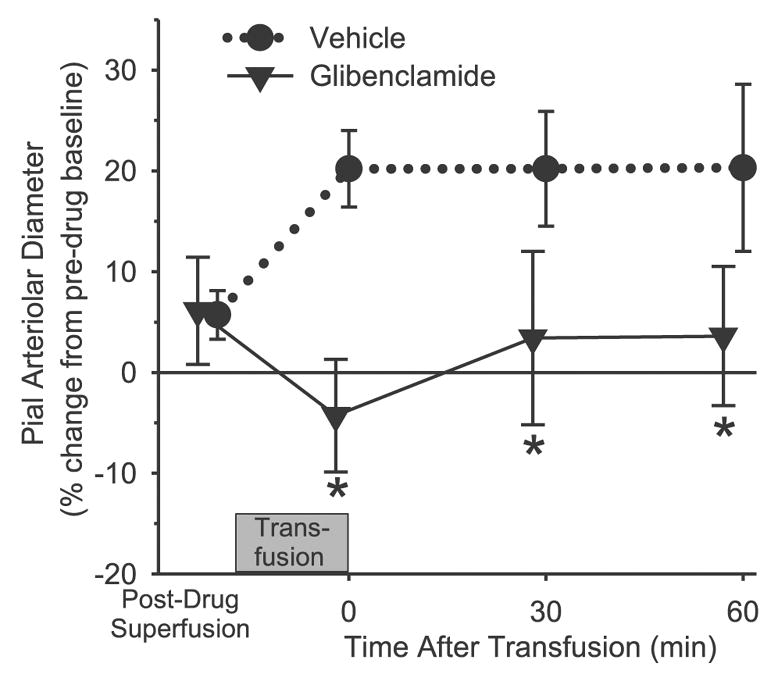
Change in pial arteriolar diameter after superfusion of the cranial window with 0.1% DMSO vehicle (n = 6) or 10 μM glibenclamide (n = 6), and at 0, 30, and 60 min after completion of an exchange transfusion of the albumin solution. Values are means ± SD and are expressed as a percentage of the baseline diameter before vehicle or drug superfusion of the window. *P < 0.05 from vehicle group.
Superfusion of the surface of the brain with 0.1 μM U-46619 1 h after transfusion reduced the diameter of pial arterioles in all groups (Fig. 4). The response in the albumin-transfused group superfused with glibenclamide is not different from the response seen in the albumin-transfused group superfused with vehicle. Likewise, the constrictor responses to U-46619 in the hemoglobin-transfused groups superfused with the various inhibitors are not significantly different from that seen in the hemoglobin-transfused group superfused with vehicle.
Fig. 4.
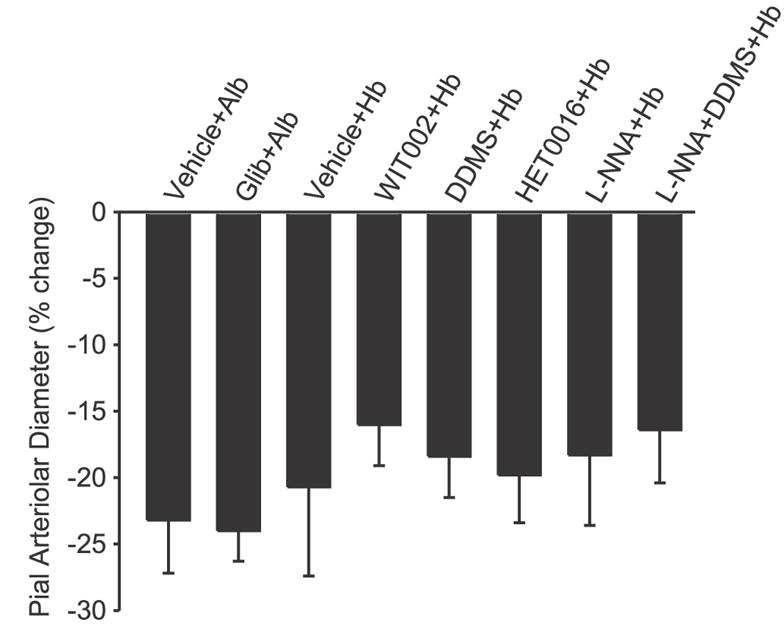
Percent change in pial arteriolar diameter during superfusion of the thromboxane analog U-46619 (1 μM) 1 h after completion of the exchange transfusion with the albumin (Alb) or hemoglobin (Hb) in the various groups treated with vehicle or drugs. Values are means ± SD. There were no significant differences between the treatment groups and the respective vehicle groups.
DISCUSSION
This study examined potential mechanisms mediating the change in pial arteriolar diameter after decreases in hematocrit with and without a proportional decrease in O2-carrying capacity. The major findings were 1) that elevations in the production of 20-HETE appears to play an important role in constricting pial arterioles after decreases in hematocrit when O2-carrying capacity is maintained at near-normal levels by transfusion of a cell-free hemoglobin polymer; 2) that the expected fall in NO levels by binding to hemoglobin does not play a major role in this constrictor response; and 3) that activation of KATP channels contributes to the pial arteriolar dilation seen when hematocrit is decreased and there are a parallel decreases in the O2-carrying capacity of the blood.
The results obtained with DDMS in blocking the constrictor response to hemoglobin transfusion are similar to those obtained by using a chemically dissimilar and more selective inhibitor of the synthesis of 20-HETE, HET-0016. Moreover, similar results were obtained by superfusing the brain with an antagonist of the vasoconstrictor properties of 20-HETE, WIT-002 (5, 37). The concordant results obtained with the three distinct pharmacological probes support a role for 20-HETE in mediating the constriction of pial arteries after exchange transfusion with a hemoglobin-containing solution.
Exchange transfusion reduces hematocrit and blood viscosity and thus would normally act to increase cerebral blood flow. Because the O2-carrying capacity of the blood has been maintained by the hemoglobin solution, an increase in delivery of O2 to the brain would be expected if there was no change in vascular tone. Indeed, an improvement of O2 delivery to the brain after hypoperfusion is one of the therapeutic targets for the development of this class of compounds. The production of 20-HETE by CYP4A enzymes is directly related to the prevailing tissue Po2 levels over the normal physiological range of 20–80 Torr found in various tissues (7). The results of the present study imply that the formation of 20-HETE from arachidonic acid by CYP ω-hydroxylase in arteriole smooth muscle increases after exchange transfusion with the hemoglobin-containing solution and that the elevated levels of 20-HETE contributes to arteriolar constriction to prevent overoxygenation when blood viscosity is decreased without a proportional reduction in O2-carrying capacity. Thus the mechanism involved in this autoregulation to an imposed change in blood viscosity is similar to that proposed for 20-HETE in mediating autoregulation of cerebral blood flow after increases in arterial blood pressure (5).
In addition to its O2 dependency, the formation of 20-HETE in isolated cerebral arteries is augmented by increases in transmural pressure (5). Thus one needs to consider whether an increase in transmural pressure might also provide the initial stimulus for pial arteriolar constriction by a 20-HETE -dependent mechanism. At constant aortic pressure, pial arteriolar intravascular pressure would increase if the effect decreased blood viscosity resulted in a disproportionately greater reduction in the pressure drop across extraparenchymal resistance vessels compared with that across the intraparenchymal resistance vessels. However, because the major effect of reducing hematocrit is on blood viscosity at low shear rates present in the microcirculation, one would not anticipate a disproportionate effect on the macrocirculation vascular resistance. Indeed, servo-null measurements did not demonstrate a significant change in pial arteriolar intravascular pressure with reduced hematocrit in rats (12). Thus an increase in pial arteriolar transmural pressure is unlikely to be the major contributor to the observed 20% constriction after hemoglobin exchange transfusion.
Another consideration is that 20-HETE production is strongly inhibited by NO. For example, NO donors have been found to open calcium-activated potassium channels and produce cerebral vasodilation, and part of this response depends on NO inhibition of ω-hydroxylase activity rather than increases in cGMP (1, 30). Indeed, we found that the decrease in baseline pial arteriolar diameter seen with the NOS inhibitor L-NNA was blocked by coapplication of the ω-hydroxylase inhibitor DDMS, whereas DDMS application alone had no effect on baseline diameter. Previous work has also shown that intracisternal injection of WIT-002 had no effect on baseline cortical perfusion (37). Together, these findings suggest that tonic NO production normally suppresses basal 20-HETE formation to very low levels but that inhibition of basal NO production results in constriction that is dependent on 20-HETE synthesis.
Hemoglobin transfusion in the presence of L-NNA produced additional pial arteriolar constriction compared with L-NNA alone. Cell-free hemoglobin in plasma can scavenge NO more effectively than red cell-based hemoglobin (10, 17, 18). Mathematical models of the microcirculation indicate that the presence of hemoglobin in the plasma can theoretically scavenge sufficient NO to reduce NO concentration in vascular smooth muscle (14). Thus the constrictor response to exchange transfusion of cell-free hemoglobin could be mediated by decreases in NO sufficient either to decrease cGMP or to increase the formation of 20-HETE. Our observation that the effect of combining L-NNA with DDMS on blocking the constrictor response was the same as administering DDMS implies that the inhibitory effect of DDMS does not require the presence of NO. Thus the effect of transfusing cell-free hemoglobin is unlikely to be due to scavenging of NO by hemoglobin. Moreover, the observation that constriction was still present with L-NNA alone implies that a decrease in cGMP as a result of NO scavenging by hemoglobin plays only a minor role, if any, in the constrictor response.
Previous work in cats showed that pial arteriolar constriction to cell-free hemoglobin transfusion also persisted in the presence of L-NNA (27). In this case, however, exchange transfusion was performed with a cross-linked tetramer of hemoglobin that extravasates into renal lymph and increases arterial pressure. The increase in arterial pressure could have augmented myogenic tone, which also stimulates the formation of 20-HETE in cerebral arteries (5). In the present study, administration of the polymeric hemoglobin that does not extravasate in renal lymph (19) did not increase arterial pressure, and L-NNA still had little effect on the constrictor response to exchange transfusion, whereas inhibitors of the synthesis or actions of 20-HETE were completely effective. In aggregate, these results are consistent with the concept that cell-free hemoglobin exchange transfusion increases the formation of 20-HETE in cerebral arteries by increasing O2 availability for this O2-dependent enzyme.
Glibenclamide has been reported to attenuate the dilation of pial arterioles (29, 31) and the increase in cerebral blood flow during hypoxic hypoxia (32). Some evidence suggests that anemia can reduce O2 availability in brain, although hypotension that often accompanies hemodilution may also participate (28, 36). Decreases in Po2 in pial venules has been reported during hemodilution, in contrast to an increase in venular Po2 after exchange transfusion of cross-linked tetrameric hemoglobin at reduced hematocrit (34). The present data showing that glibenclamide blocks pial arteriolar dilation during anemia induced by albumin exchange transfusion indicate that KATP channels ordinarily contribute to an active dilatory response to anemia, possibly as a result of a hypoxic stimulus. A decrease in transmural pressure is unlikely to fully account for the observed 14% dilation because others have reported that a 5-mmHg decrease in mean arterial blood pressure, as occurred in the present study, would result in only a 2–3% dilation of rat pial arterioles (8) and that pial arteriolar intravascular pressure remained unchanged after hemodilution (12). Our results with anemia differ from those of Tomiyama et al. (32), who found that injection of glibenclamide into cisternal CSF failed to prevent the increase in blood flow induced by hemodilution. The different findings may be due to differences in methodology.
The initial constriction seen after hemoglobin transfusion gradually subsided over the 1-h observation period, although the diameter response remained significantly different from the stable dilation seen after albumin transfusion. A similar diminishment of pial arteriolar constriction was previously observed in the cat (25), indicating that the presently observed effect was not specific for the rat. In the present study, hematocrit, total blood hemoglobin concentration, arterial blood gases, and arterial blood pressure did not change over the 1-h period (data not shown) and thus did not account for the diminished constriction over time. Time-dependent dilation after hemoglobin transfusion persisted in the groups treated with the NOS and ω-hydroxylase inhibitors, although the magnitude of the dilation between the 0-min and the 60-min time point was attenuated. Persistent dilation suggests that other pathways may be partly responsible for the diminished constriction over time.
In summary, the present work demonstrates a role for 20-HETE in mediating the cerebral vasoconstrictor response to exchange transfusion with a cell-free hemoglobin polymer. The constrictor response observed at a reduced hematocrit with this O2 carrier in the absence of arterial hypertension suggests that this signaling pathway is important in a form of viscosity autoregulation that limits overoxygenation of the brain. Some effort has been directed at designing hemoglobin O2 carriers with reduced affinity for NO to minimize unwanted vasoconstriction (24, 26). The present study indicates that constriction can occur independent of NO in a vascular bed with tight endothelial junctions and that this constriction acts to overcome the decrease in blood viscosity. Others have proposed that designing a hemoglobin-based solution with high viscosity and high O2 affinity will overcome arteriolar constriction in beds such as skeletal muscle, which can reduce capillary perfusion (13, 33). Whether elevations in 20-HETE also play a role in the vascular response to cell-free hemoglobin transfusion in other vascular beds remains to be determined.
Acknowledgments
The authors are grateful to Tzipora Sofare for fine editorial assistance in preparing this manuscript.
Footnotes
DISCLOSURES
E. Bucci and the University of Maryland are holders of a patent on the zero-link bovine hemoglobin polymer used in this study.
R. C. Koehler and H. Kwansa are paid consultants to Oxyvita, holder of the licensing rights to the zero-link bovine hemoglobin polymer. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
GRANTS
This work was supported by National Institutes of Health Grants NS-38684 and HL-59996.
References
- 1.Alonso-Galicia M, Hudetz AG, Shen H, Harder DR, Roman RJ. Contribution of 20-HETE to vasodilator actions of nitric oxide in the cerebral microcirculation. Stroke. 1999;30:2727–2734. doi: 10.1161/01.str.30.12.2727. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 3.Asano Y, Koehler RC, Kawaguchi T, McPherson RW. Pial arteriolar constriction to α2-adrenergic agonist dexmedetomidine in the rat. Am J Physiol Heart Circ Physiol. 1997;272:H2547–H2556. doi: 10.1152/ajpheart.1997.272.6.H2547. [DOI] [PubMed] [Google Scholar]
- 4.Asano Y, Koehler RC, Ulatowski JA, Traystman RJ, Bucci E. Effect of cross-linked hemoglobin transfusion on endothelial-dependent dilation in feline pial arterioles. Am J Physiol Heart Circ Physiol. 1998;275:H1313–H1321. doi: 10.1152/ajpheart.1998.275.4.H1313. [DOI] [PubMed] [Google Scholar]
- 5.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60 – 65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 6.Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol Heart Circ Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 7.Harder DR, Narayanan J, Birks EK, Liard JF, Imig JD, Lombard JH, Lange AR, Roman RJ. Identification of a putative microvascular oxygen sensor. Circ Res. 1996;79:54–61. doi: 10.1161/01.res.79.1.54. [DOI] [PubMed] [Google Scholar]
- 8.Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am J Physiol Heart Circ Physiol. 1984;246:H17–H24. doi: 10.1152/ajpheart.1984.246.1.H17. [DOI] [PubMed] [Google Scholar]
- 9.Hirata T, Koehler RC, Kawaguchi T, Brusilow SW, Traystman RJ. Impaired pial arteriolar reactivity to hypercapnia during hyperammonemia depends on glutamine synthesis. Stroke. 1996;27:729–736. doi: 10.1161/01.str.27.4.729. [DOI] [PubMed] [Google Scholar]
- 10.Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang C, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci USA. 2001;98:11771–11776. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudak ML, Jones MD, Jr, Popel AS, Koehler RC, Traystman RJ, Zeger SL. Hemodilution causes size-dependent constriction of pial arterioles in the cat. Am J Physiol Heart Circ Physiol. 1989;257:H912–H917. doi: 10.1152/ajpheart.1989.257.3.H912. [DOI] [PubMed] [Google Scholar]
- 12.Hurn PD, Traystman RJ, Shoukas AA, Jones MD., Jr Pial microvascular hemodynamics in anemia. Am J Physiol Heart Circ Physiol. 1993;264:H2131–H2135. doi: 10.1152/ajpheart.1993.264.6.H2131. [DOI] [PubMed] [Google Scholar]
- 13.Intaglietta M. Microcirculatory basis for the design of artificial blood. Microcirculation. 1999;6:247, 258. [PubMed] [Google Scholar]
- 14.Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282:H2245–H2253. doi: 10.1152/ajpheart.00972.2001. [DOI] [PubMed] [Google Scholar]
- 15.Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, Hudetz AG, Schulte ML, Zagorac D, Harder DR, Roman RJ. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H1556–H1565. doi: 10.1152/ajpheart.00924.2001. [DOI] [PubMed] [Google Scholar]
- 16.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxy-eicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 17.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci USA. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Samouilov A, Lancaster JR, Jr, Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 19.Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93:1479–1486. doi: 10.1152/japplphysiol.00191.2002. [DOI] [PubMed] [Google Scholar]
- 20.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muizelaar JP, Bouma GJ, Levasseur JE, Kontos HA. Effect of hematocrit variations on cerebral blood flow and basilar artery diameter in vivo. Am J Physiol Heart Circ Physiol. 1992;262:H949–H954. doi: 10.1152/ajpheart.1992.262.4.H949. [DOI] [PubMed] [Google Scholar]
- 22.Muizelaar JP, Wei EP, Kontos HA, Becker DP. Mannitol causes compensatory cerebral vasoconstriction and vasodilation in response to blood viscosity changes. J Neurosurg. 1983;59:822–828. doi: 10.3171/jns.1983.59.5.0822. [DOI] [PubMed] [Google Scholar]
- 23.Muizelaar JP, Wei EP, Kontos HA, Becker DP. Cerebral blood flow is regulated by changes in blood pressure and in blood viscosity alike. Stroke. 1986;17:44–48. doi: 10.1161/01.str.17.1.44. [DOI] [PubMed] [Google Scholar]
- 24.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 25.Rebel A, Ulatowski JA, Kwansa H, Bucci E, Koehler RC. Cerebrovascular response to decreased hematocrit: effect of cell-free hemoglobin, plasma viscosity, and CO2. Am J Physiol Heart Circ Physiol. 2003;285:H1600–H1608. doi: 10.1152/ajpheart.00077.2003. [DOI] [PubMed] [Google Scholar]
- 26.Resta TC, Walker BR, Eichinger MR, Doyle MP. Rate of NO scavenging alters effects of recombinant hemoglobin solutions on pulmonary vasoreactivity. J Appl Physiol. 2002;93:1327–1336. doi: 10.1152/japplphysiol.00175.2002. [DOI] [PubMed] [Google Scholar]
- 27.Sampei K, Ulatowski JA, Asano Y, Kwansa H, Bucci E, Koehler RC. Role of nitric oxide scavenging in vascular response to cell-free hemoglobin transfusion. Am J Physiol Heart Circ Physiol. 2005;289:H1191–H1201. doi: 10.1152/ajpheart.00251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schacterle RS, Ribando RJ, Adams JM. A model of brain arteriolar oxygen and carbon dioxide transport during anemia. J Cereb Blood Flow Metab. 1993;13:872–880. doi: 10.1038/jcbfm.1993.109. [DOI] [PubMed] [Google Scholar]
- 29.Shankar V, Armstead WM. Opioids contribute to hypoxia-induced pial artery dilation through activation of ATP-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 1995;269:H997–H1002. doi: 10.1152/ajpheart.1995.269.3.H997. [DOI] [PubMed] [Google Scholar]
- 30.Sun CW, Falck JR, Okamoto H, Harder DR, Roman RJ. Role of cGMP versus 20-HETE in the vasodilator response to nitric oxide in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2000;279:H339–H350. doi: 10.1152/ajpheart.2000.279.1.H339. [DOI] [PubMed] [Google Scholar]
- 31.Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994;74:1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- 32.Tomiyama Y, Brian JE, Jr, Todd MM. Cerebral blood flow during hemodilution and hypoxia in rats: role of ATP-sensitive potassium channels. Stroke. 1999;30:1942–1947. doi: 10.1161/01.str.30.9.1942. [DOI] [PubMed] [Google Scholar]
- 33.Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 2003;285:H1411–H1419. doi: 10.1152/ajpheart.00307.2003. [DOI] [PubMed] [Google Scholar]
- 34.Vovenko E, Golub A, Pittman R. Microvascular Po2 and blood velocity measurements in rat brain cortex during hemodilution with a plasma expander (Hespan) and a hemoglobin-based oxygen carrier (DCLHb) Adv Exp Med Biol. 2003;540:215–220. doi: 10.1007/978-1-4757-6125-2_30. [DOI] [PubMed] [Google Scholar]
- 35.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 36.Watanabe M, Harada N, Kosaka H, Shiga T. Intravital microreflectometry of individual pial vessels and capillary region of rat. J Cereb Blood Flow Metab. 1994;14:75–84. doi: 10.1038/jcbfm.1994.12. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]


