Abstract
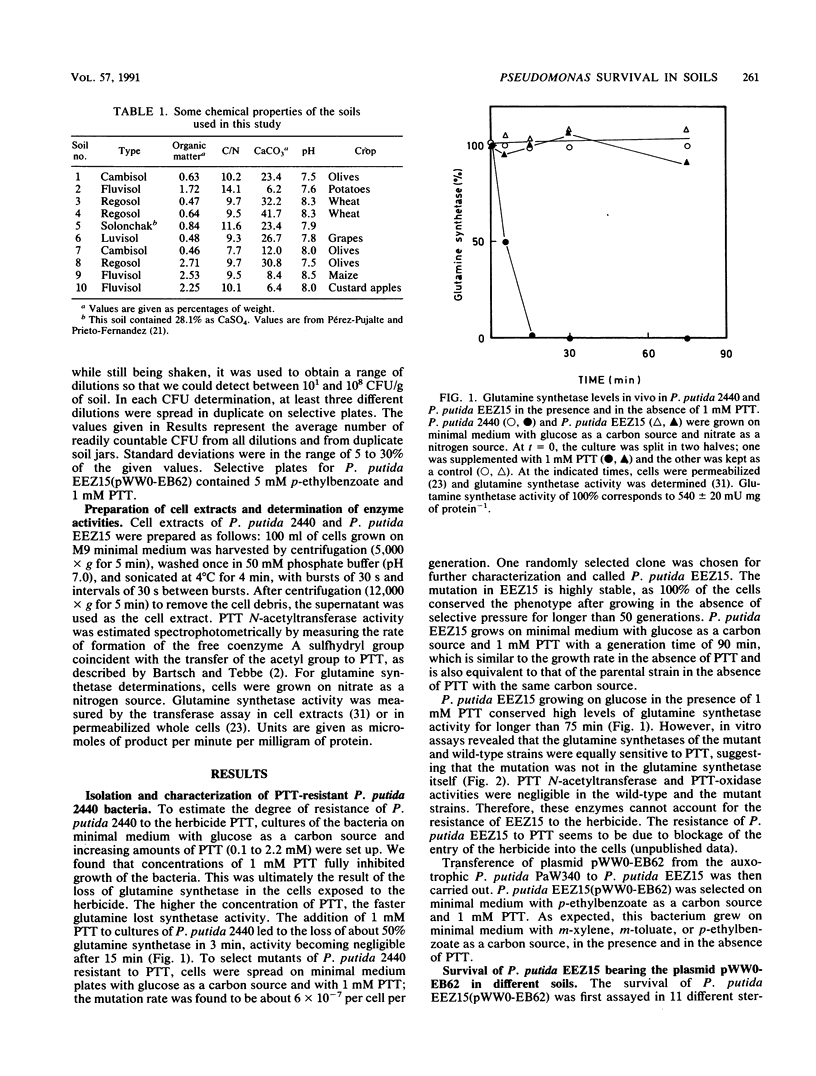
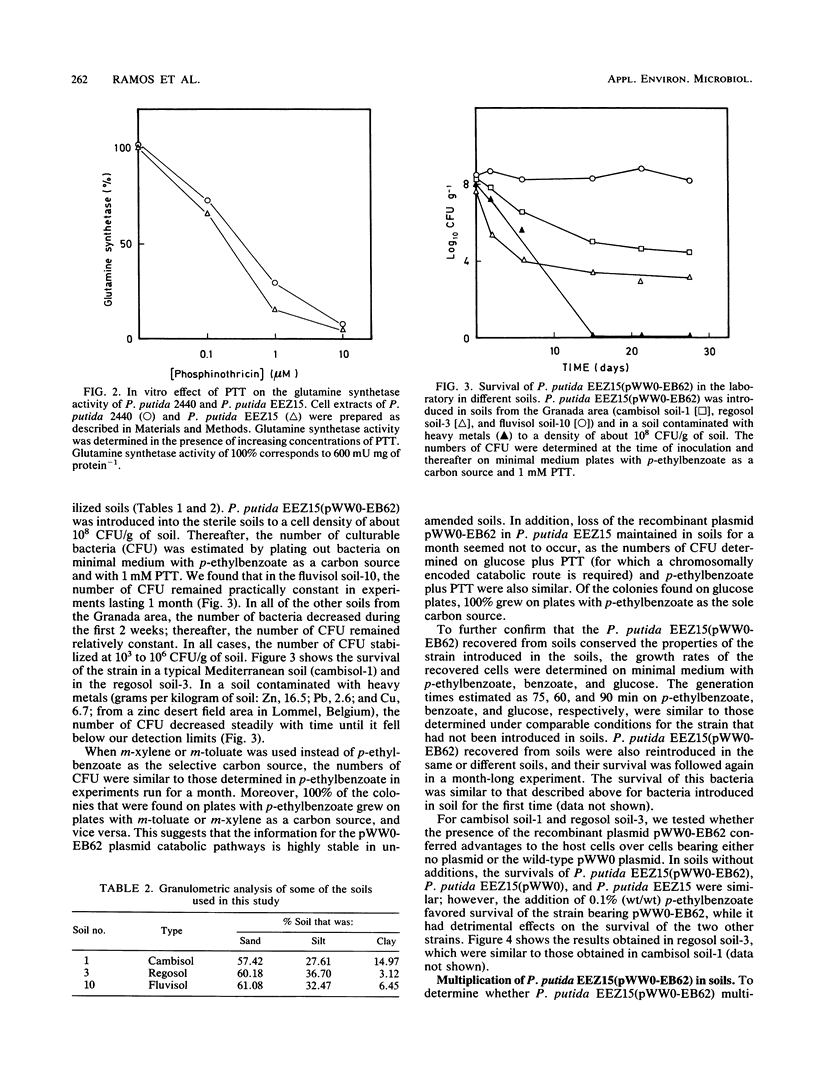
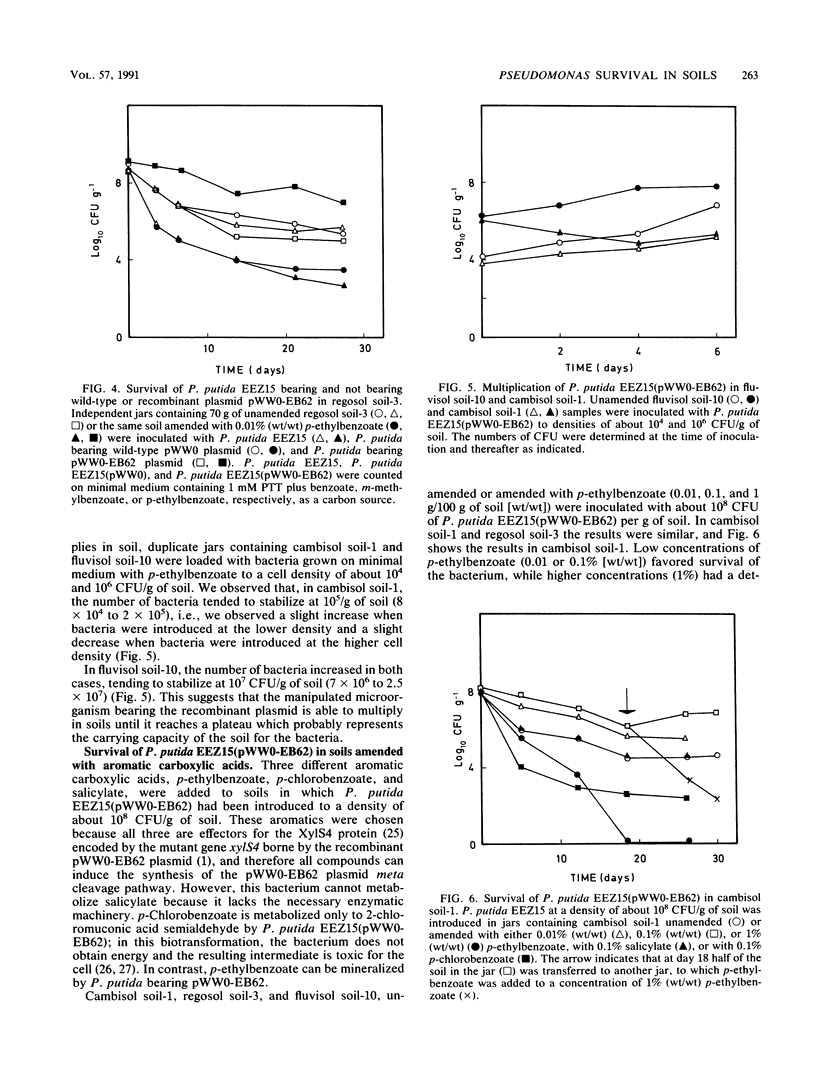
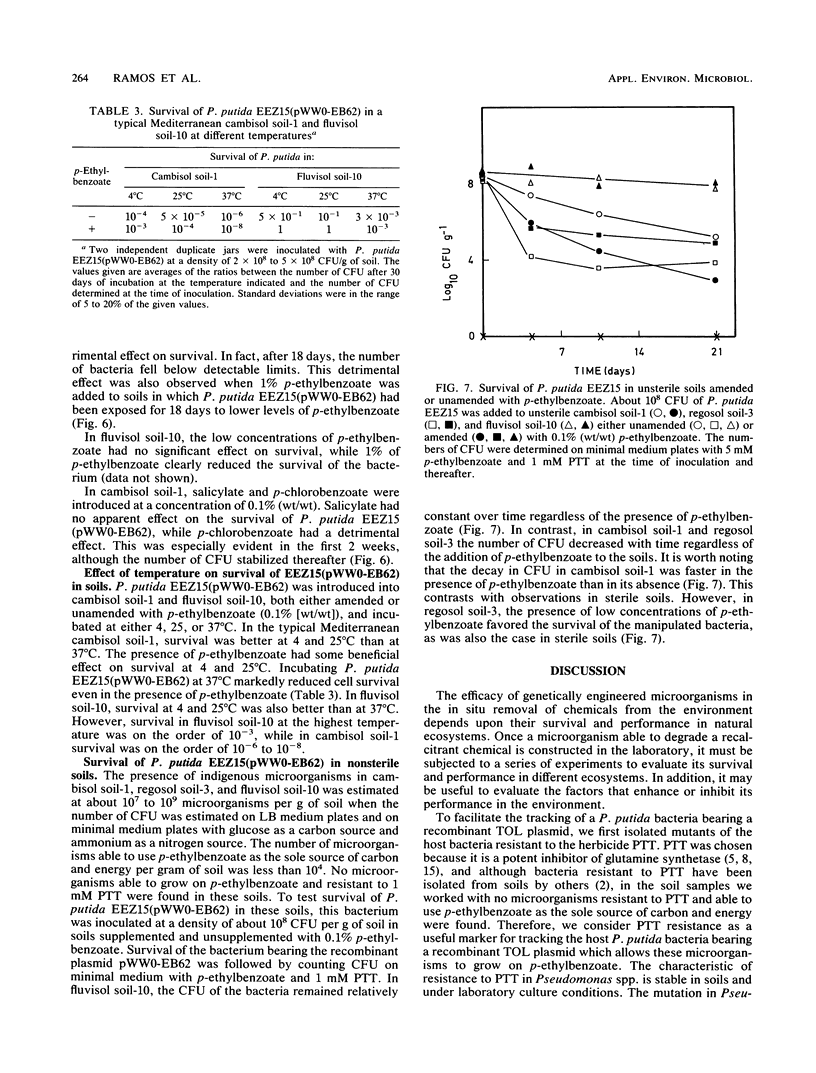
Pseudomonas putida EEZ15(pWW0-EB62) is a phosphinothricin (PPT)-resistant strain with a recombinant TOL plasmid which allows the strain to grow on p-ethylbenzoate. The survival of this strain in sterile agricultural soils depends on the physicochemical properties of the soil. The recombinant pWW0-EB62 plasmid and its catabolic functions were stable for periods of up to 1 month in bacteria introduced in unamended soils and only conferred selective advantage to the host bacteria without the plasmid or with the natural pWW0 plasmid when the soils were amended with low amounts of p-ethylbenzoate. The addition to soils of aromatics that are cometabolized by P. putida EEZ15(pWW0-EB62) had a detrimental effect on the survival of the bacteria, whereas low amounts of aromatics that are not metabolized by this bacterium had no effect on their survival. Survival of P. putida EEZ15(pWW0-EB62) was better at 4 and 25 degrees C than at 37 degrees C. The host bacterium carrying the recombinant pWW0-EB62 plasmid was established in unsterile soils.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abril M. A., Michan C., Timmis K. N., Ramos J. L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989 Dec;171(12):6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch K., Tebbe C. C. Initial steps in the degradation of phosphinothricin (glufosinate) by soil bacteria. Appl Environ Microbiol. 1989 Mar;55(3):711–716. doi: 10.1128/aem.55.3.711-716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentjen S. A., Fredrickson J. K., Van Voris P., Li S. W. Intact soil-core microcosms for evaluating the fate and ecological impact of the release of genetically engineered microorganisms. Appl Environ Microbiol. 1989 Jan;55(1):198–202. doi: 10.1128/aem.55.1.198-202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciafardini G., Marotta B. Use of the Most-Probable-Number Technique To Detect Polymyxa betae (Plasmodiophoromycetes) in Soil. Appl Environ Microbiol. 1989 May;55(5):1273–1278. doi: 10.1128/aem.55.5.1273-1278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin F. C., Bagdasarian M., Bagdasarian M. M., Timmis K. N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulthorpe R. R., Wyndham R. C. Survival and activity of a 3-chlorobenzoate-catabolic genotype in a natural system. Appl Environ Microbiol. 1989 Jun;55(6):1584–1590. doi: 10.1128/aem.55.6.1584-1590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. M., Mallory L. M., Alexander M. Reasons for possible failure of inoculation to enhance biodegradation. Appl Environ Microbiol. 1985 Oct;50(4):977–983. doi: 10.1128/aem.50.4.977-983.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeenes D. J., Williams P. A. Excision and integration of degradative pathway genes from TOL plasmid pWW0. J Bacteriol. 1982 Apr;150(1):188–194. doi: 10.1128/jb.150.1.188-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. M., Alexander M. Bacterial inhibitors in lake water. Appl Environ Microbiol. 1986 Jul;52(1):114–118. doi: 10.1128/aem.52.1.114-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach P. R., Zeyer J., Reineke W., Knackmuss H. J., Timmis K. N. Enzyme recruitment in vitro: use of cloned genes to extend the range of haloaromatics degraded by Pseudomonas sp. strain B13. J Bacteriol. 1984 Jun;158(3):1025–1032. doi: 10.1128/jb.158.3.1025-1032.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L. N., Sinclair J. L., Mallory L. M., Alexander M. Fate in model ecosystems of microbial species of potential use in genetic engineering. Appl Environ Microbiol. 1982 Sep;44(3):708–714. doi: 10.1128/aem.44.3.708-714.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure N. C., Weightman A. J., Fry J. C. Survival of Pseudomonas putida UWC1 containing cloned catabolic genes in a model activated-sludge unit. Appl Environ Microbiol. 1989 Oct;55(10):2627–2634. doi: 10.1128/aem.55.10.2627-2634.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Gonzalez-Carrero M., Timmis K. N. Broad-host range expression vectors containing manipulated meta-cleavage pathway regulatory elements of the TOL plasmid. FEBS Lett. 1988 Jan 4;226(2):241–246. doi: 10.1016/0014-5793(88)81431-5. [DOI] [PubMed] [Google Scholar]
- Ramos J. L., Stolz A., Reineke W., Timmis K. N. Altered effector specificities in regulators of gene expression: TOL plasmid xylS mutants and their use to engineer expansion of the range of aromatics degraded by bacteria. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8467–8471. doi: 10.1073/pnas.83.22.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Timmis K. N. Experimental evolution of catabolic pathways of bacteria. Microbiol Sci. 1987 Aug;4(8):228–237. [PubMed] [Google Scholar]
- Ramos J. L., Wasserfallen A., Rose K., Timmis K. N. Redesigning metabolic routes: manipulation of TOL plasmid pathway for catabolism of alkylbenzoates. Science. 1987 Jan 30;235(4788):593–596. doi: 10.1126/science.3468623. [DOI] [PubMed] [Google Scholar]
- Reineke W., Jeenes D. J., Williams P. A., Knackmuss H. J. TOL plasmid pWW0 in constructed halobenzoate-degrading Pseudomonas strains: prevention of meta pathway. J Bacteriol. 1982 Apr;150(1):195–201. doi: 10.1128/jb.150.1.195-201.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Construction of haloaromatics utilising bacteria. Nature. 1979 Feb 1;277(5695):385–386. doi: 10.1038/277385a0. [DOI] [PubMed] [Google Scholar]
- Rojo F., Pieper D. H., Engesser K. H., Knackmuss H. J., Timmis K. N. Assemblage of ortho cleavage route for simultaneous degradation of chloro- and methylaromatics. Science. 1987 Dec 4;238(4832):1395–1398. doi: 10.1126/science.3479842. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Shields M. S., Tedford E. T., Breen A., Hooper S. W., Sirotkin K. M., Davis J. W. Application of DNA-DNA colony hybridization to the detection of catabolic genotypes in environmental samples. Appl Environ Microbiol. 1985 May;49(5):1295–1303. doi: 10.1128/aem.49.5.1295-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Alexander M. Effects of dissolved organic carbon and second substrates on the biodegradation of organic compounds at low concentrations. Appl Environ Microbiol. 1985 Apr;49(4):822–827. doi: 10.1128/aem.49.4.822-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C., Morgan J. A., Pickup R. W., Jones J. G., Saunders J. R. Differential regulation of lambda pL and pR promoters by a cI repressor in a broad-host-range thermoregulated plasmid marker system. Appl Environ Microbiol. 1989 Apr;55(4):771–777. doi: 10.1128/aem.55.4.771-777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


