Abstract
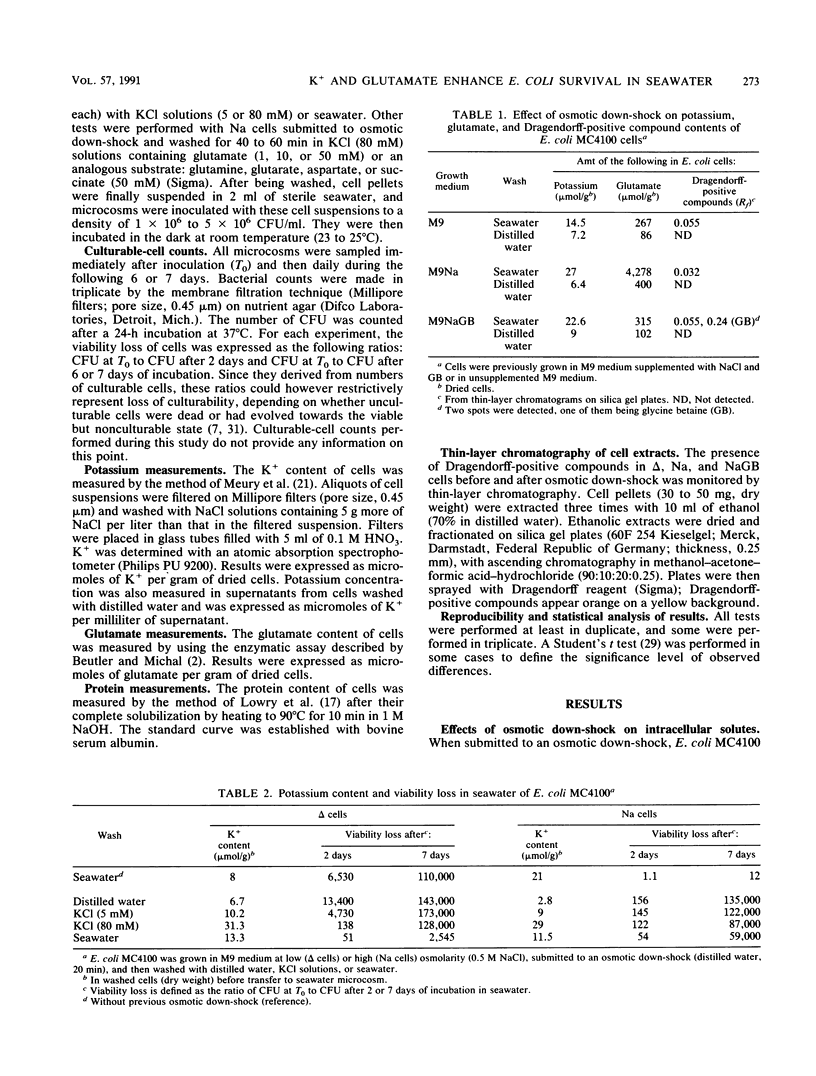
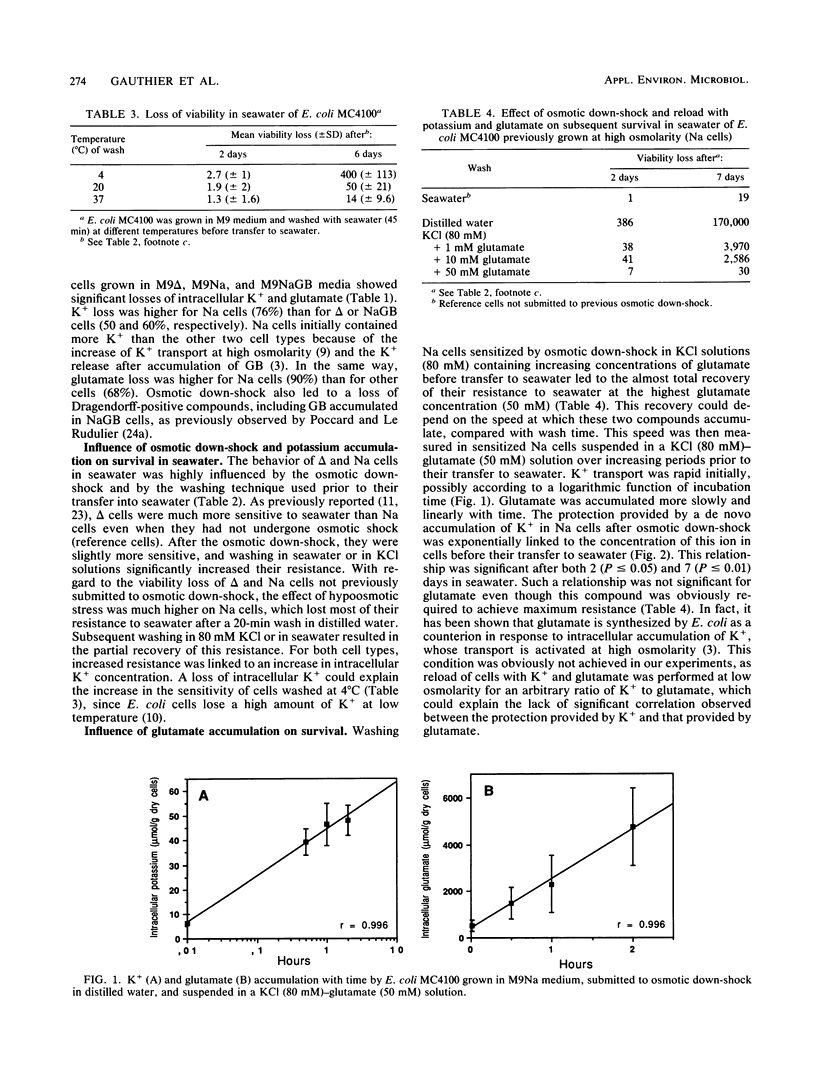
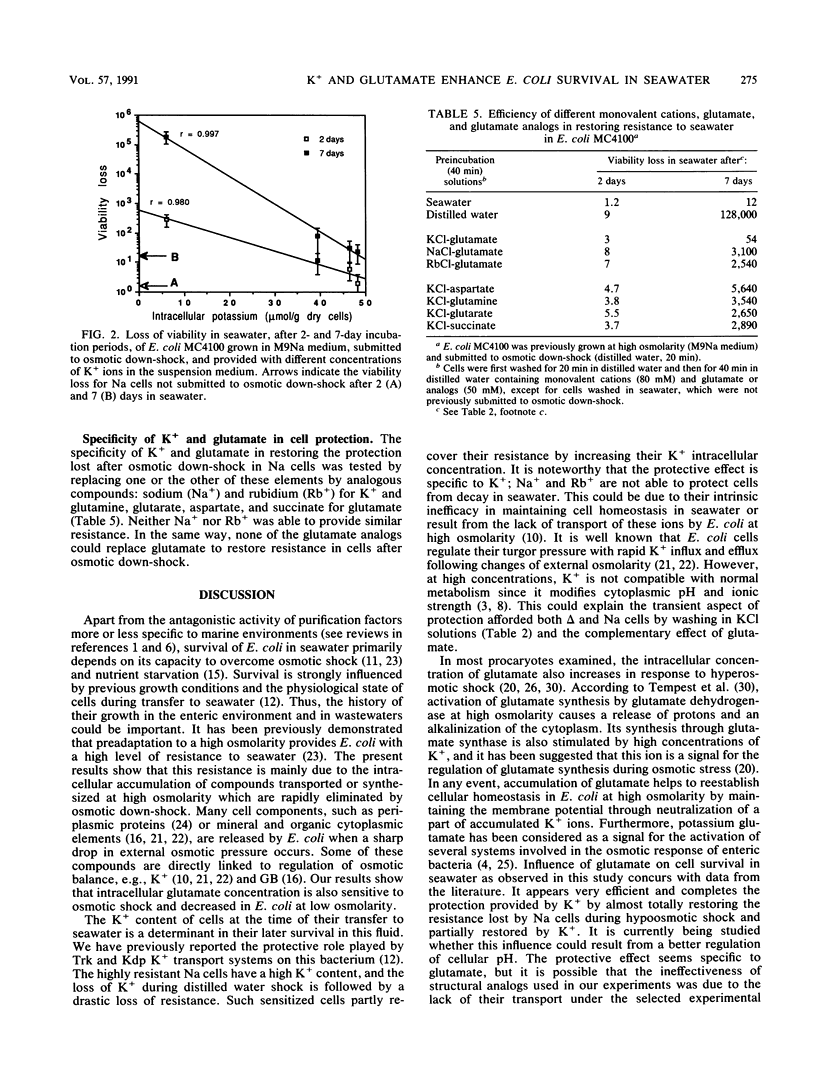
The high resistance of Escherichia coli grown in saline media to seawater was suppressed by an osmotic down-shock. The shock released several molecules into the medium, including potassium, glutamate, and glycine betaine when cells were previously grown in the presence of this osmolyte. Incubation of such sensitized cells in a solution containing K+ (80 mM) and glutamate (50 mM) at pH 7.4 restored their resistance to seawater up to a level close to that observed initially. The protective effect was partly due to the rapid accumulation of K+; a significant exponential relationship between intracellular concentration of K+ and resistance to seawater was observed. Glutamate was accumulated more slowly and progressively completed the action of K+. These data emphasize the specific influence of potassium glutamate on osmotically stressed E. coli cells. They confirm that regulation of osmotic pressure and, probably, of intracellular pH strongly enhances survival of E. coli in seawater. Osmotic fluctuations in waters carrying enteric bacteria from intestines to seawater, together with variations in their K+ and amino acid contents, could modify the ability of cells to survive in marine environments. These results demonstrate the need to strictly control conditions (K+ content, temperature) used to wash cells before their transfer to seawater microcosms. They suggest that the K+ and glutamate contents of media in which E. coli cells are transported to the sea can influence their subsequent survival in marine environments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth I. R., Cairney J., Sutherland L., Higgin C. F. Enteric bacteria and osmotic stress: an integrated homeostatic system. Soc Appl Bacteriol Symp Ser. 1988;17:35S–49S. [PubMed] [Google Scholar]
- Booth I. R., Higgins C. F. Enteric bacteria and osmotic stress: intracellular potassium glutamate as a secondary signal of osmotic stress? FEMS Microbiol Rev. 1990 Jun;6(2-3):239–246. doi: 10.1111/j.1574-6968.1990.tb04097.x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Colwell R. R. From counts to clones. Soc Appl Bacteriol Symp Ser. 1987;16:1S–6S. doi: 10.1111/j.1365-2672.1987.tb03606.x. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M. J., Le Rudulier D. Survival in seawater of Escherichia coli cells grown in marine sediments containing glycine betaine. Appl Environ Microbiol. 1990 Sep;56(9):2915–2918. doi: 10.1128/aem.56.9.2915-2918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M. J., Munro P. M., Breittmayer V. A. Influence of prior growth conditions on low nutrient response of Escherichia coli in seawater. Can J Microbiol. 1989 Mar;35(3):379–383. doi: 10.1139/m89-058. [DOI] [PubMed] [Google Scholar]
- Ghoul M., Bernard T., Cormier M. Evidence that Escherichia coli accumulates glycine betaine from marine sediments. Appl Environ Microbiol. 1990 Feb;56(2):551–554. doi: 10.1128/aem.56.2.551-554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Meury J., Kepes A. The regulation of potassium fluxes for the adjustment and maintenance of potassium levels in Escherichia coli. Eur J Biochem. 1981 Sep;119(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05589.x. [DOI] [PubMed] [Google Scholar]
- Meury J., Robin A., Monnier-Champeix P. Turgor-controlled K+ fluxes and their pathways in Escherichia coli. Eur J Biochem. 1985 Sep 16;151(3):613–619. doi: 10.1111/j.1432-1033.1985.tb09148.x. [DOI] [PubMed] [Google Scholar]
- Munro P. M., Gauthier M. J., Breittmayer V. A., Bongiovanni J. Influence of osmoregulation processes on starvation survival of Escherichia coli in seawater. Appl Environ Microbiol. 1989 Aug;55(8):2017–2024. doi: 10.1128/aem.55.8.2017-2024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R. M., Prince W. S., Bremer E., Villarejo M. In vitro reconstitution of osmoregulated expression of proU of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1153–1157. doi: 10.1073/pnas.86.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey B., Cayley D. S., Mossing M. C., Kolka C., Anderson C. F., Farrar T. C., Record M. T., Jr Variability of the intracellular ionic environment of Escherichia coli. Differences between in vitro and in vivo effects of ion concentrations on protein-DNA interactions and gene expression. J Biol Chem. 1987 May 25;262(15):7157–7164. [PubMed] [Google Scholar]
- Roth W. G., Leckie M. P., Dietzler D. N. Osmotic stress drastically inhibits active transport of carbohydrates by Escherichia coli. Biochem Biophys Res Commun. 1985 Jan 16;126(1):434–441. doi: 10.1016/0006-291x(85)90624-2. [DOI] [PubMed] [Google Scholar]
- Roth W. G., Leckie M. P., Dietzler D. N. Restoration of colony-forming activity in osmotically stressed Escherichia coli by betaine. Appl Environ Microbiol. 1988 Dec;54(12):3142–3146. doi: 10.1128/aem.54.12.3142-3146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]


