Abstract
Primary, secondary, and tertiary alcohols as well as phenols and carbohydrates are efficiently transformed into the corresponding 2-tetrahydrofuranyl ethers by a combination of Mn(0) powder and CCl4 in tetrahydrofuran.
Keywords: Protecting group, Manganese, Radical reaction, Tetrahydrofuran
Despite its well-established reputation1 as a versatile, orthogonal2 protecting group, the 2-tetrahydrofuranyl (THF) ether is often disregarded in favor of its homologous relative, the tetrahydropyranyl (THP) ether. This is due, in large measure, to limitations in the extant procedures for its introduction, i.e., the required reagents not commercially available, corrosive, incompatible with sensitive functionality, and/or unstable.2-11 To address these issues, we introduced a convenient protocol utilizing CrCl2 and CCl4 in tetrahydrofuran.12 However, the high costs of CrCl2, the need for a large excess of reagent, and chromium’s toxicity spurred us to seek a more economic and environmentally benign alternative.
Initially, a panel of readily available, eco-friendly metals was evaluated for their ability to promoted the 2-tetrahydrofuranylation of n-octanol (1) under a standard set of reaction conditions (0.4 M in THF, 65°C, 15 h, 1.5 equiv of CCl4). Fe(0) and Zn(0) furnished only modest yields of 213 (73% and 63%, respectively). Mg(0), with the largest reduction potential of all the metals tested, gave rise to a disappointing 5% of the desired THF ether. The most consistent results were obtained with Mn(0) powder.14 Just 1.5 equivalents of Mn(0) provided an excellent yield of 2 (Table 1, entry 1); fewer equivalents of Mn(0) led to portionately lower yields of 2.
Table 1.
preparation of THF ethers.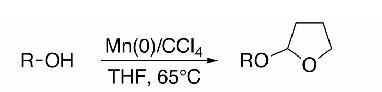
| Entry | Alcohol | THF Ether | Time(h) | yield(%) |
|---|---|---|---|---|
| 1 | 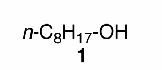 |
 |
15 | 96 |
| 2 |  |
 |
15 | 99a |
| 3 | 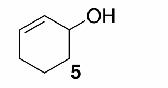 |
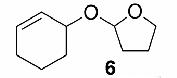 |
7 | 85a |
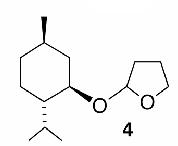
| 4 | 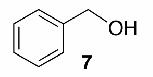 |
 |
4 | 93 |
| 5 | 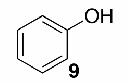 |
 |
6 | 91 |
| 6 | 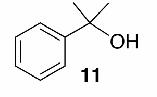 |
 |
6 | 88a |
| 7 | 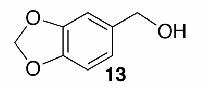 |
 |
14 | 90 |
| 8 | 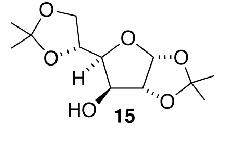 |
 |
6 | 99a,b |
| 9 | 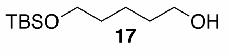 |
 |
3 | 96 |
| 10 | 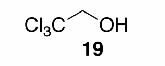 |
 |
4 | 88b |
| 11 | 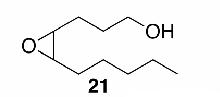 |
 |
13 | 85a |
| 12 | 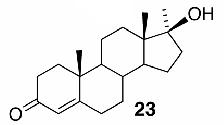 |
 |
8 | 91a,b |
∼1:1 diastereomeric mixture by NMR analysis.
2 equiv each of Mn(0) and ccl4 were used.
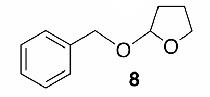
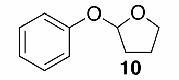
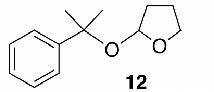
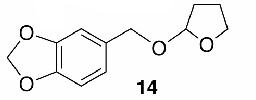
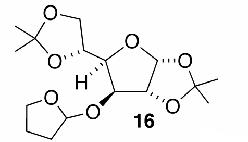
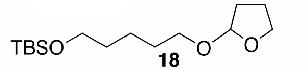
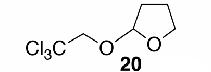
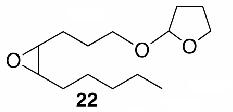
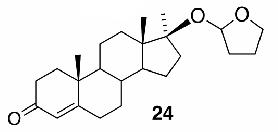
Likewise, secondary12 (entry 2), allylic15 (entry 3), and benzylic12 (entry 4) THF ethers were easily obtained from alcohols 3, 5, and 7, respectively. Less reactive hydroxyls such as phenol (9) and the highly hindered dimethylphenylcarbinol 11 were transformed without complication into their THF derivatives 1012 (entry 5) and 1212 (entry 6), respectively. Importantly, a variety of common funtionality proved compatible with the standard reaction conditions. For instance, methylenedioxy (entry 7), acetonide (entry 8), and silyl (entry 9) groups were all well tolerated and led accordingly to THF ethers 14,12 16,15 and 18.15 The successful protection of labile 1,1,1-trichloride 1912, acid sensitive epoxide 21, and α,β-unsaturated steroidal ketone 23, all in excellent yields, are especially notable.15
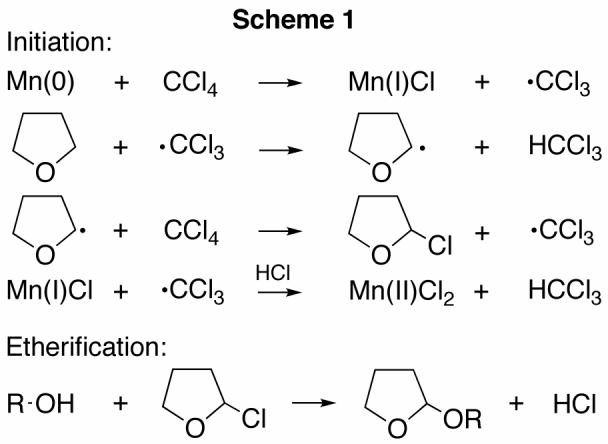
The mechanism of the tetrahydrofuranylation most like parallels that of the CrCl2-mediated reaction,12 i.e., single electron transfer from Mn(0)16 to CCl4 during the initiation phase generates the well-known trichloromethyl radical (Scheme 1). This radical subsequently abstracts a hydrogen atom from the tetrahydrofuran methylene adjacent to oxygen in the first step of the propagation phase forming chloroform and a heteroatom stabilized radical that is chlorinated by a second molecule of CCl4. The newly evolved trichloromethyl radical can either propagate the reaction via hydrogen atom abstraction from another equivalent of tetrahydrofuran or is further reduced and in the process consumes the HCl produced during the etherification step.
General Procedure: CCl4 (0.145 mL, 1.5mmol, 1.5 equiv) was added via syringe to a stirring suspension of alcohol (1 mmol, 1.0 equiv) and Mn(0) powder14 (83 mg, 1.5 mmol, 1.5 equiv) in anhydrous THF (3 mL) under an argon atmosphere and then warmed to 65°C. A white precipitate of MnCl2 accumulated during the course of the reaction. After the times indicated in Table 1, the reaction mixture was cooled to room temperature, diluted with ether (20 mL), filtered through a pad of silica gel, and the filter cake was washed with ether. In most cases, the residue after concentration in vacuo required no further purification, but if necessary, was passed over a SiO2 column to give the corresponding THF ether in indicated yields (Table 1).
In summary, an operationally simple, inexpensive, and efficient method to make THF ethers has been developed. Its mild reaction conditions and general tolerance of most functional groups make it widely applicable in the synthesis of complex molecules.
Acknowledgments
Financial support provided by the Robert A. Welch Foundation, NIH (GM31278, DK38226), and Ministèredáláguá à l’Enseignement supárieur et à la Recherche.
References
- 1.Greene TW, Wuts PGM. Protective Groups in Organic Chemistry. Wiley; New York: 1999. pp. 57–58. Chapter 2. [Google Scholar]
- 2. THF ethers can be selectively cleaved in the presence of THP ethers, e.g., see references 1 and 3.
- 3.Kruse CG, Broekhof NLJM, van der Gen A. Tetrahedron Lett. 1976;20:1725–1728. 2-Chlorotetrahydrofuran. [Google Scholar]
- 4.Sosnovsky G. J. Org. Chem. 1965;33:2441. Dihydrofuran. [Google Scholar]
- 5.Hon YS, Lee CF. Tetrahedron Lett. 1999;40:2389–2392. [Google Scholar]
- 6.Kruse CG, Poels EK, Jonkers FL, van der Gen A. J. Org. Chem. 1978;43:3548. Acetal exchange. [Google Scholar]
- 7.Maione AM, Romeo A. Synthesis. 1987:250. THF/ceric ammonium nitrate. [Google Scholar]
- 8.Jung JC, Choi HC, Kim YH. Tetrahedron Lett. 1993;34:3581–3584. THF/tetrabutylammonium peroxydisulfate. [Google Scholar]
- 9.Yu B, Hui Y. Syn. Commun. 1995;25:2037–2042. p-TsCl/NaH/THF. [Google Scholar]
- 10.Barks JM, Gilbert BC, Parsons AF, Upeandran B. Tetrahedron Lett. 2000;41:6249. BrCCl3/2,4,6-collidine. [Google Scholar]
- 11.Ochiai M, Sueda T. Tetrahedron Lett. 2004;45:3557. tert-Butylperoxy-λ3-iodane/CCl4. [Google Scholar]
- 12.Baati R, Valleix A, Mioskowski C, Barma DK, Falck JR. Org. Lett. 2000;2:485–487. doi: 10.1021/ol991332f. [DOI] [PubMed] [Google Scholar]
- 13.Seiders JR, II, Wang L, Floreancig PE. J. Am. Chem. Soc. 2003;125:2406. doi: 10.1021/ja029139v. [DOI] [PubMed] [Google Scholar]
- 14. Mn(0) powder was purchased from Aldrich Chem. (99%,-325 mesh). While it could be weighed and handled without special precautions, it was stored under an inert atmosphere to help retain its full reactivity.
- 15. Spectral data for 6 (∼1:1 diastereomeric mixture): 1H NMR (300 MHz, CDCl3) δ 5.86-5.68 (m, 4H), 5.32 (dd, J = 4.2, 2.1 Hz, 1H), 5.29 (dd, J = 4.2, 1.8 Hz, 1H), 4.18-4.11 (m, 2H), 3.94-3.82 (m, 4H), 2.02-1.84 (m, 20H); 13C NMR (75 MHz, CDCl3) δ 131.0, 130.6, 129.2, 127.9, 103.3, 102.0, 70.9, 69.7, 66.8 (2C), 32.9, 32.7, 30.5, 28.4, 25.3, 25.2, 23.7 (2C), 19.6, 19.4; HRMS (CI, CH4) calcd for C10H17O2 (M + 1) m/e 169.1228, found 169.1230. Compound 16 (∼1:1 diastereomeric mixture): H NMR (400 MHz, CDCl3) δ 5.87 (d, J = 4.0 Hz, 1H), 5.84 (d, J = 3.2 Hz, 1H), 5.31 (t, J = 2.8 Hz, 1H), 5.24 (br s, 1H), 4.60 (d, J = 3.2 Hz, 1H), 4.51 (d, J = 3.6 Hz, 1H), 4.31 (d, J = 3.6 Hz, 1H), 4.26-4.16 (m, 4H), 4.11-3.99 (m, 2H), 3.98-3.86 (m, 6H), 1.99-1.83 (m, 8H), 1.49 (s, 6H), 1.42 (s, 6H), 1.34 (s, 6H), 1.31 (s, 6H); 13C NMR (50 MHz, CDCl3) δ 111.8, 109.0, 108.8, 105.3, 101.2, 84.2, 82.4, 81.1, 80.2, 76.4, 72.8, 72.7, 67.4, 67.20, 67.16, 67.1, 32.4, 27.0, 26.9, 26.7, 26.3, 25.5, 23.4, 23.0. Compound 18 (∼1:1 diastereomeric mixture): 1H NMR (400 MHz, CDCl3) δ 5.09 (d, J = 3.2 Hz, 1H), 3.88-3.81 (m, 2H), 3.68-3.60 (m, 3H), 3.40-3.34 (m, 1H), 2.01-1.79 (m, 4H), 1.61-1.54 (m, 4H), 0.91 (s, 9H), 0.03 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 103.9, 67.1, 66.8, 63.1, 32.4, 29.7, 26.3, 26.1 (3C), 23.6, 18.5,-5.1 (2C); HRMS (CI, CH4) calcd for C15H33O3Si (M + 1) m/e 289.2199, found 289.2198. Compound 22: 1:1 diastereo mixture; 1H NMR (300 MHz, CDCl3): δ = 5.38 (dd, J = 4.2Hz, 1.8Hz, 1H), 5.13 (dd, J = 4.2Hz, 1.8Hz, 1H), 3.89-3.59 (m, 10H), 3.50-3.41 (m, 2H), 1.84-1.71 (m, 16H), 1.58-1.20 (m, 18H), 0.81-0.77 (m, 6H); 13C NMR (75 MHz, CDCl3): δ = 104.0, 103.0, 82.6, 80.0, 78.1, 77.8, 77.7, 68.5, 68.0, 67.0, 66.8, 32.6, 32.5, 32.2, 32.1, 32.0, 30.8, 28.5, 27.3, 26.2, 26.0, 25.6, 25.2, 23.7, 22.8, 22.7, 14.1 (2C); HRMS (CI, CH4) calcd for C14H27O3 (M + 1) m/e 243.1960, found 243.1960. Compound 24 (∼1:1 diastereomeric mixture): 1H NMR (300 MHz, CDCl3) δ 5.72 (s, 2H), 5.39-5.37 (m, 1H), 5.34-5.32 (m, 1H), 3.94-3.88 (m, 2H), 3.79-3.74 (m, 2H), 2.50-2.22 (m, 8H), 2.19-1.24 (m, 26H), 1.24 (s, 3H), 1.22 (s, 3H), 1.21 (s, 3H), 1.19 (s, 3H), 1.02-0.86 (m, 4H), 0.85 (s, 3H), 0.84 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 199.8 (2C), 171.1, 171.6, 124.0 (2C), 100.0, 99.6, 86.2, 85.8, 66.9, 66.7, 54.0 (2C), 49.9, 49.8 (2C), 46.6, 46.1, 38.8, 36.6, 36.5, 36.4, 36.1, 35.9 (2C), 34.1 (2C), 33.6, 33.5, 33.0 (2C), 32.6, 32.0 (2C), 31.7, 24.1 (2C), 23.7, 23.3, 23.2, 22.5, 20.9, 20.8, 17.6 (2C), 14.4, 14.2; HRMS (CI, CH4) calcd for C24H37O3 (M + 1) m/e 373.2743, found 373.2740.
- 16.Takai K, Ueda T, Ikeda N, Ishiyama T, Matsushita H. Bull. Chem. Soc. Jpn. 2003;76:347–353. [Google Scholar]


