Abstract
We previously reported that perinatal exposure to hypergravity affects cerebellar structure and motor coordination in rat neonates. In the present study, we explored the hypothesis that neonatal cerebellar structure and motor coordination may be particularly vulnerable to the effects of hypergravity during specific developmental stages. To test this hypothesis, we compared neurodevelopment, motor behavior and cerebellar structure in rat neonates exposed to 1.65 G on a 24-ft centrifuge during discrete periods of time: the 2nd week of pregnancy [gestational day (G) 8 through G15; group A], the 3rd week of pregnancy (G15 through birth on G22/G23; group B), the 1st week of nursing [birth through postnatal day (P) 6; group C], the 2nd and 3rd weeks of nursing (P6 through P21; group D), the combined 2nd and 3rd weeks of pregnancy and nursing (G8 through P21; group E) and stationary control (SC) neonates (group F). Prenatal exposure to hypergravity resulted in intrauterine growth retardation as reflected by a decrease in the number of pups in a litter and lower average mass at birth. Exposure to hypergravity immediately after birth impaired the righting response on P3, while the startle response in both males and females was most affected by exposure during the 2nd and 3rd weeks after birth. Hypergravity exposure also impaired motor functions, as evidenced by poorer performance on a rotarod; while both males and females exposed to hypergravity during the 2nd and 3rd weeks after birth performed poorly on P21, male neonates were most dramatically affected by exposure to hypergravity during the second week of gestation, when the duration of their recorded stay on the rotarod was one half that of SC males. Cerebellar mass was most reduced by later postnatal exposure. Thus, for the developing rat cerebellum, the postnatal period that overlaps the brain growth spurt is the most vulnerable to hypergravity. However, male motor behavior is also affected by midpregnancy exposure to hypergravity, suggesting discrete and sexually dimorphic windows of vulnerability of the developing central nervous system to environmental perturbations.
Keywords: Hypergravity, Rat, Cerebellum, Developmental vulnerability, Motor Functions
1. Introduction
Accumulating evidence suggests that intrauterine and neonatal exposure to environmental factors modulates gene expression and affects the course of the CNS development. While a number of chemicals (Nguon et al., 2005a) have been shown to affect the developing CNS structure and function, our own previous studies suggested that the developing CNS may also be vulnerable to altered gravity (Sajdel-Sulkowska et al., 2001; Nguon et al., 2004; Sulkowski et al., 2004; Nguon et al., 2005b).
Impairment in motor coordination observed in rat neonates developing under hypergravity was accompanied by changes in cerebellar structure. These observations are not surprising, since even adult organisms respond to altered gravity. The adult CNS may show an adjustment to altered gravity in the form of altered behavior, as indicated by observation that the majority of astronauts experience changes in motor coordination during their initial exposure to weightlessness in space and the initial period following their return to Earth (Tafforin, 1990; Layne et al., 1997; Reschke et al., 1998; Yates et al., 2003). On the other hand, the response of the developing CNS to altered gravity is more dramatic, encompassing extensive changes in both CNS structure and function as suggested by observations in rats that the prenatal exposure to microgravity affects the righting response (Bruce and Fritzsch, 1997) and motor and equilibrium behavior (Aizikov and Markin, 1981) that are associated with structural changes in the brain (Azikov and Markin, 1981). Animals in space show decreased fiber arborization, retarded synaptogenesis (Bruce and Fritzsch, 1997), and neuronal degeneration in various brain regions (Savel’ev et al., 1998). Exposure to both hypergravity and microgravity has detrimental effects on the locomotor development in young rats (Bouet et al., 2004a). While it is difficult to perform extensive developmental studies in space, hypergravity paradigms, using centrifuges in lieu of spaceflight, have provided a useful environment in which to predict the effects of microgravity on biological organisms (Serova, 1985).
Hypergravity-exposed pups exhibit decreased exploratory behavior (Thullier et al., 2002) and rats exposed to hypergravity show changes in synaptic organization (Gimenez y Ribotta et al., 1998). However, few studies have directly and systematically correlated changes in motor behavior with structural abnormalities in the cerebellum or compared the effect of altered gravity across the developmental spectrum. The identification of critical windows of vulnerability to environmental manipulations, including changes in gravitational loading, may allow for interventions to prevent long-term impairment of CNS structure and function in the context of extended space flights. Therefore, a thorough understanding of the impact of altered gravity on neurodevelopment is of paramount importance for prolonged missions in space.
In designing the present study, we hypothesized that neonatal cerebellar structure and motor coordination may be particularly vulnerable to the effect of hypergravity during critical developmental stages. Furthermore, on the basis of previous observations of the sexually dimorphic response of rat neonates to hypergravity, we inferred that the timing of vulnerable periods may be sexually dimorphic.
We explored this hypothesis by separately comparing neurodevelopment, motor behavior and cerebellar structure independent of each other, in male and female rat neonates exposed to 1.65 G on a 24-ft centrifuge during discrete periods of time: the 2nd week of pregnancy [gestational day (G) 8, through G15; group A], the 3rd week of pregnancy (G15 through birth on G22/G23; group B), the 1st week of nursing [birth through postnatal day (P) 6; group C)], the 2nd and 3rd weeks of nursing (P6 through P21; group D), the combined 2nd and 3rd weeks of pregnancy and nursing (G8 through P21; group E) and stationary control (SC) neonates (group F).
2. Methods
2.1 Animals and treatment
Timed-pregnant Sprague-Dawley dams were shipped from Taconic Farms (Germantown, NY) to NASA Ames Research Center (ARC) at gestational day (G)2 (G1 defined as the first day after males and females are co-housed and on which the female has either a sperm plug or a sperm-positive vaginal smear). Dams were housed individually under standard vivarium conditions (12-hr light cycle, lights on at 6 AM and off at 6 PM, at 21-24°C). Standard laboratory rat chow and water were available ad libitum.
On G7, dams were weight-matched, deselected if not pregnant, and assigned to one of the following groups: A (exposed to 1.65G from G8 to G15, n=17), B (exposed to 1.65G from G15 to birth, n=14), C (exposed to 1.65G from birth to P6, n=15), D (exposed to 1.65G from P6 to P21, n=6), E (exposed to 1.65G from G8 to P21, n=18) and F (SC, n=32). Period A corresponds to the time of Purkinje cell birth and thus exposure to hypergravity during this time would be likely to affect the Purkinje cell number as indeed is the case (Sajdel-sulkowska et al., 2005). Period B, corresponds to the period of Purkinje cell migration. Period C overlaps with the brain growth-spurt period (P4-P10; Heaton et al., 1999) and period D overlaps with both the period of brain growth spurt and cerebellar granule cell migration. We were somewhat limited in the selection of experimental groups by the space-limitation of the centrifuge rotunda.
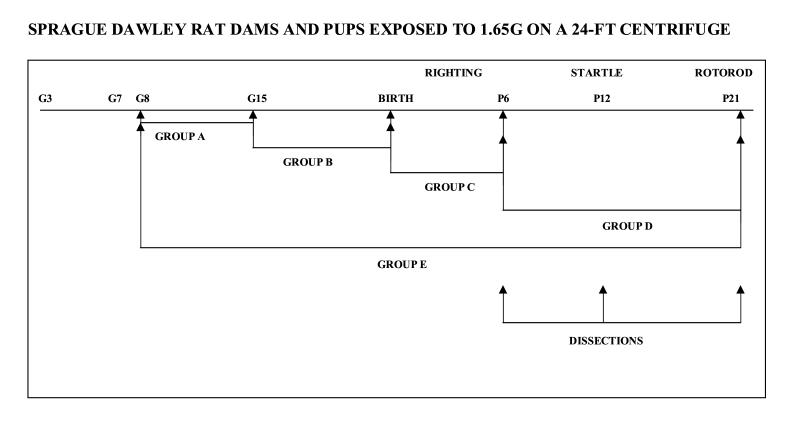
The dams were then transferred to the ARC 24-ft centrifuge rotunda. Both HG and SC dams were placed one per cage, and two or four cages were loaded per cab; all animals were exposed to the same environment. On G8, continuous centrifugation was initiated, with brief daily stops for checks of animal health and data collection both before and after the animals’ birth. The centrifugation was carried out at 17.6 rpm and the cabs were placed 136.5 inches from the center, resulting in hypergravity of 1.65 G. The duration of the stops in all experimental groups was 30 min before births and 90 min following births. The experimental diagram is shown in Fig. 1.
Figure 1.
Experimental paradigm.
Maternal mass and food consumption were recorded daily starting on G3. Body mass and mortality among neonates were recorded daily from birth until they were sacrificed by decapitation on P12 and P21. Their brains were then rapidly removed and the cerebella were dissected from the brain stem, weighed, frozen in methyl butane (Schmitz et al., 2000), and stored at -80°C for further analysis.
2.2 Developmental and behavioral tests
The righting response, expressed in terms of the time required for a rat to right itself when placed in the supine position, was determined daily from P2 to P4.
The startle response, expressed in terms of a pup’s ability to respond with head movement to the sound of snapping fingers, was first observed on P10 and tested daily on P10-P15 in a subset of randomly selected litters. The results were expressed as the percentage of males or females in the entire experimental group, and were analyzed on P14.
Eye opening, expressed in terms of the total number of eyes open per litter, was first observed on P11 and recorded daily on P11-P15 in a subset of randomly selected litters. The results were expressed as a percentage of total eyes opened in males or females per litter.
Motor coordination was measured on P21 by a rotarod test according to the procedure described earlier (Auvray et al., 1989; Caston et al., 1998). The pups, placed perpendicular to the horizontally rotating cylinder with their heads facing against the direction of rotation, had to move forward to maintain equilibrium and remain on the cylinder. Each neonate was subjected to one trial on a cylinder rotating at 24 rpm. The length of time the animal remained on the rotating cylinder was recorded (in seconds) and the performances were compared among pups in different groups.
2.3. Tissue collection
On P12 and P21, live SC and HG litters were euthanized by decapitation without anesthesia. The neonatal brains were then rapidly removed and the cerebella were dissected out (Nguon et al., 2004; Sulkowski et al., 2004). The cerebella were weighed by two independent investigators and rapidly frozen for further analysis.
2.5 Statistical Analysis
When applicable, a two-way ANOVA was run to determine the relationship between treatment and sex. If a statistically significant interaction was found, a two-sample t-test was carried out and the values were adjusted for multiple comparisons by the Bonferroni correction. All values are reported as mean ± S.E.M.; for all statistical tests the 0.05 level of confidence was accepted for statistical significance.
3. Results
Selection of gravity level.
In selecting the level of gravity exposure we were guided by the higher magnitude of changes in the parameters studied at higher gravity while taking into account the increase in neonatal mortality observed at higher gravity level (Ladd et al., 2005). We have selected 1.65G for the current experiment because of our previous data indicating the overall mortality of pups exposed to 1.65G during gestation and lactation was 34.1±2.5% while the mortality of pups exposed to 1.75HG was 47.8±0.9% (Ladd et al., 2005).
Litter size.
Dams exposed to 1.65G during pregnancy gave birth to a smaller litter than SC dams. Exposure during the 3rd week (group B) had a greater effect on litter size than exposure during the 2nd week (group A) only, but combined exposure during both the 2nd and 3rd weeks of pregnancy resulted in the smallest litter (Table 1). ANOVA showed a significant treatment effect F(5,81)=3.48, p=0.0067.
Table 1.
| GROUP A n=17 litters | GROUP B n=14 litters | GROUP C n=15 litters | GROUP D n=6 litters | GROUP E n=18 | GROUP F n=32 litters | |
|---|---|---|---|---|---|---|
| bbbbbAVERAGE LITTER SIZE(# NEONATES | 11.5±0.73 | 10.06±0.82 | 12.31±0.49 | 13.0±0.63 | 9.8±0.85 | 12.7±0.40 |
| MORTALITY (%) | 12.9±7.2 | 12.9±7.2 | 53.3±10.7 | 2.4±2.4 | 23.2±5.1 | 11.1±6.5 |
| CANNIBALISM (%) | 2.6±1.6 | 8.7±6.2 | 29.8±7.0 | 2.4±2.4 | 18.8±4.7 | 8.6±6.0 |
| AVERAGE BODY MASS AT BIRTH OF MALE NEONATES (GRAMS±S.E.M. | 6.93±0.138 | 6.04±0.156 | 7.17±0.136 | 6.90±0.225 | 6.63±0.177 | 7.25±0.127 |
| AVERAGE BODY MASS AT BIRTH OF FEMALE NEONATES (GRAMS±S.E.M. | 6.69±0.108 | 5.64±0.137 | 6.84±0.119 | 6.75±0.198 | 6.34±0.165 | 6.90±0.129 |
Effect of exposure to 1.65G during different developmental periods on litter size, mortality, cannibalism, and neonatal body mass at birth (mean ± S.E.M.). Litter size was most strongly affected by combined exposure during pregnancy and nursing (group E; p=0.0067); greatest mortality (p=0.0002) and cannibalism (p=0.053) of neonates was observed among pups exposed to 1.65G immediately after birth (group C); neonatal body mass was most affected by exposure during the 2nd trimester of pregnancy (group A; p<0.0001).
Neonatal mortality.
The rate of mortality in offspring exposed to 1.65G was dependent on the period of exposure to hypergravity. The highest mortality (53.3%±10.7) was observed among neonates in group C, born to SC mothers and transferred to hypergravity at birth (Table 1). Mortality among the neonates exposed to hypergravity during the 2nd (group A) or the 3rd (group B) week of gestation was 12.9%±7.2 and 11.2%±6.6, which almost doubled in neonates exposed during both the 2nd and the 3rd weeks of gestation (23.2%±5.1). ANOVA revealed a strong influence of timing of exposure to hypergravity, with F(5,80)=5.55, p=0.0002. One of the most unexpected findings was the mortality rate in group C (pups transferred to hypergravity at birth). Though the mortality rate was low during the first 48 hours after the onset of centrifugation, the death rate increased dramatically at 72 to 144 hours after the onset of centrifugation. The deaths coincided with the so-called critical developmental period identified in rats between P4 and P9. The duration of attrition was also dependent on the period of exposure to hypergravity with ANOVA showing a strong dependence with F(5,81)=9.97, p<0.0001. The average duration of attrition in group C was 4.4 days as compared to 1.7 days in the SC group; in group E the average duration of attrition was 2.4 days. Further analysis indicated that a significant proportion of deaths was due to cannibalism (Table 1); ANOVA showed a strong effect of hypergravity, with F(5,81)=3.62, p=0.053. More than half (29.8±7.0% of 53.3±10.7%) of the neonates born to SC dams and transferred to the centrifuge at birth were cannibalized. It is possible that dams, nutritionally challenged by the demands of nursing, consumed the weakest pups.
Neonatal body mass at birth.
Exposure to 1.65G during different pregnancy periods had specific effects on neonatal body mass at birth (Table 1). Exposure to hypergravity during the 3rd week of pregnancy (group B) resulted in the greatest decrease in neonatal body mass at birth in both males and females, while exposure during the 2nd week (group A) had very little effect. Body mass of male offspring exposed to hypergravity during the 3rd week (group B) was reduced by 16.5% compared with SC pups; body mass of female neonates exposed to hypergravity during the same period was reduced by 18.3% compared with SC pups. The combined exposure to hypergravity during 2nd and 3rd weeks of pregnancy had less effect on neonatal body mass at birth than exposure during the 3rd week alone. ANOVA showed a significant effect of treatment F(5,156)=18.94, p<0.0001 and sex effect F(1,156)=9.97, p=0.0019.
Neonatal growth.
The effect of exposure to 1.65G during different developmental periods had specific effects on neonatal body mass and neonatal growth (Fig. 2). Exposure to hypergravity after birth had a more dramatic effect on neonatal body mass on P6, P12 and P21. Exposure to hypergravity during the first week after birth (group C) resulted in a decreased body mass on P6 when the body mass of male pups was decreased by 23.6% and that of female pups was decreased by 16.6%. However, both male and female pups recovered by P21. Exposure to hypergravity during the 2nd and 3rd postnatal week (group D) resulted in the greatest decrease in neonatal body mass in both male and female pups, and the decrease was persistent on P21 when male pups attained 74.1% of SC body mass and female neonates attained 75.5 % of SC body mass. Exposure to 1.65 G during both pregnancy and nursing had no effect on neonatal body mass on P21. ANOVA showed an effect of period of exposure F(5,275)=21.13, p<0.0001, age F(2,275)=1937.3, p<0.0001 and postnatal age and treatment F(10,275)=9.61, p<0.0001.
Figure 2.
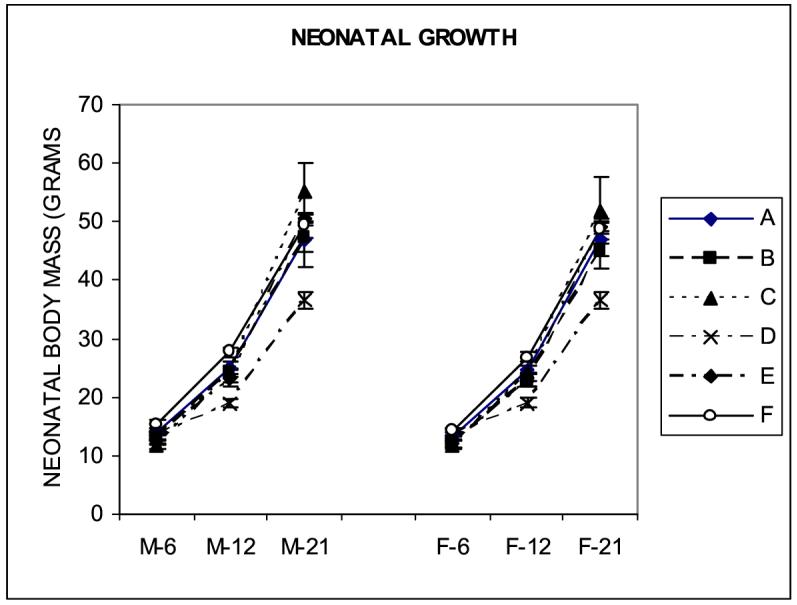
Effect of exposure to 1.65G on neonatal growth, expressed in grams of body mass (mean ± S.E.M.). Neonatal body mass was affected more by postnatal exposure (p<0.0001).
Righting response.
The time required for pups to right themselves when placed in a supine position on P3 was affected by exposure to hypergravity, depending on the period of exposure (Fig. 3); ANOVA showed a strong effect of hypergravity with F(4,320)=9.63, p<0.0001.
Figure 3.
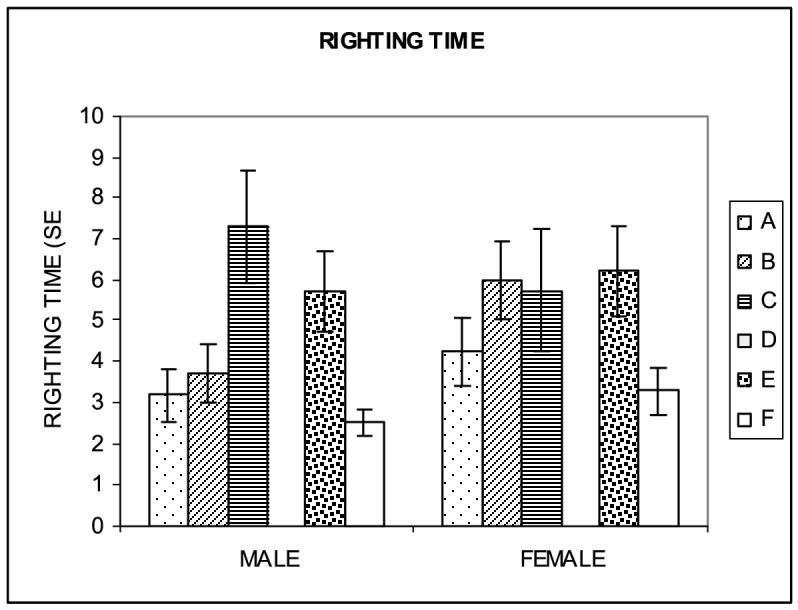
Righting time, expressed in seconds needed by neonates to right themselves when placed in a supine position (mean ± S.E.M.) Hypergravity exposure increased righting time in both male and female neonates with the most dramatic effect observed in males exposed to 1.65G immediately after birth (group C; p<0.0001).
Righting time increased most dramatically in neonates born to SC dams and transferred onto the centrifuge after birth (group C). In male HG neonates, the rightinge time was almost triple that in SC male pups and in female neonates it was more than double that in SC female pups. Exposure during the first postnatal week (group C) affected the righting time more in males than in females (Fig. 3).
Startle response.
Startle response was tested daily on P11-P13 in representative litters from each group. There was a significant F(5,58)=4.77, p=0.001 effect of hypergravity on the developmental onset of the startle response to a loud acoustic stimulus (finger snapping); onset of the startle response was delayed both in male and female pups exposed to 1.65G, as determined by the percentage of pups in each group responding to sound on a particular day. However, the delay differed depending on the period of exposure to hypergravity and was most pronounced in neonates exposed to hypergravity postnatally. On P11, none of the 1.65G pups in groups C or E showed a startle response, whereas 55% of male and 51% of female SC pups did. On P12 (Fig. 4), the delay was most pronounced for males in group D (10.3% pups responded), and for females in group C (25% pups responded), while 100% of male pups and 85.7% of female pups in group F (SC) responded. ANOVA revealed the main effects of hypergravity exposure F(5,58)=4.77, p=0.001. On P13, the startle response was most profoundly delayed in group D where only 29.7% male pups responded.
Figure 4.
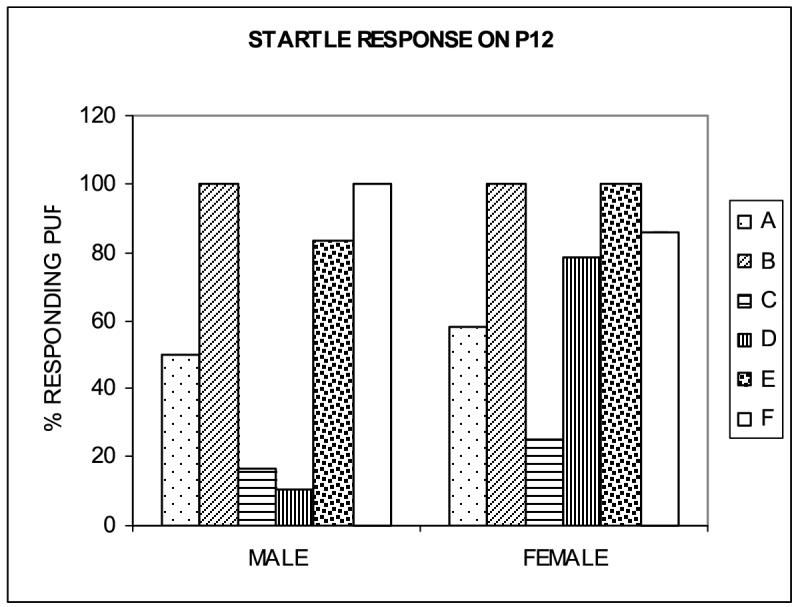
Startle response. The onset of the startle response to a loud acoustic stimulus expressed in terms of the percentage of pups in each group responding to the sound (mean ± S.E.M.) was delayed in HG male and female neonates with males most affected by hypergravity exposure during both the 1st (group C) and 2nd and 3rd weeks of nursing (group D); females were most affected by exposure to hypergravity during the first week of nursing (group C; p=0.001).
Eye opening.
Eye opening was recorded on P12-P15 in all litters in each group. We did not observe statistically significant hypergravity-induced differences in eye opening, although compared to control male pups on P14, 30% fewer male pups exposed to hypergravity during the 2nd and 3rd week after birth had their eyes open.
Rotarod test of motor behavior.
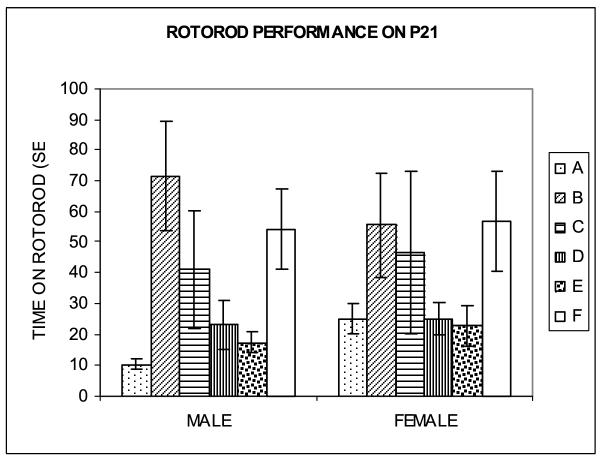
Male and female SC and HG neonates were tested on a rotarod, starting on P12 and P15 through P21. On each test day, only naive representative pups of each group were tested and the speed of the rotarod varied with age. On P12 and P15 through P18 pups were tested at incremental speeds (4-40 rpm/5 min); on P19-P20 pups were tested at a constant speed of 28 rpm; and on P21 pups were tested at a constant speed of 36 rpm. Rotarod testing on P15-P20 suggested a trend towards a shorter time on the rotating drum (i.e. rats fell off the rotating drum earlier) among hypergravity-exposed rat neonates F(4,655)=2.27, p=0.061, with male pups in groups A, B, C and E being most affected as compared to pups in group F (SC) and females in groups A, C and E being most affected as compared to pups in group F (SC). On P21, both male and female HG pups in all groups except for B performed worse than SC pups on the rotarod test as evidenced by a considerably shorter period before falling from the drum (Fig.5). ANOVA revealed the main effect of hypergravity exposure F(5,317)=5.42, p<0.001, but the effect was dependent on the period of exposure and sex. In male pups, this latency was decreased by 80.8% in group A, 24.4% in group C, 57.3% in group D, and 67.7% in group E compared with group F (SC); the decrease in groups A, D and E was statistically significant (p<0. 050). Male pups in group B stayed on the rotarod longer, but the increase was not statistically significant. In female pups the latency was decreased by 55.9% in group A, 18.4% in group C, 56.2% in group D, and 60.4% in group E; the decreases in groups A, D and E were statistically significant (p<0. 050). Female pups in group B had an increased rotarod time, but the increase was not statistically significant. The difference in performance on the rotarod test was more pronounced in pups exposed to higher gravity levels during the 2nd week of pregnancy or the 2nd and the 3rd weeks of nursing, and was most pronounced in male pups exposed to 1.65G during the 2nd week of pregnancy, among which latency before falling was 5 times shorter than in SC pups.
Figure 5.
Performance on the rotarod test on P21. Hypergravity exposure impaired performance on the rotarod as reflected in a shorter time (in seconds; mean ± S.E.M.) before falling from the rotating drum. Motor behavior was most affected by exposure to hypergravity during the 2nd and 3rd postnatal weeks (group D). The most dramatic negative effect on rotarod performance was observed in male offspring exposed to hypergravity during the 2nd week of pregnancy (group A; p<0.05).
Cerebellar mass.
As compared separately in male and female neonates on P12 and P21 (Table 2), cerebellar mass was decreased in 1.65G-exposed neonates, but the effect was strongly dependent on the developmental stage of exposure. While neonates exposed to 1.65G during fetal development on P21 showed close-to-normal cerebellar mass, exposure to 1.65G during postnatal development resulted in a significant decrease in cerebellar mass on both P12 and P21. On P12 we observed a 16.1% decrease in male and 20% decrease in female cerebellar mass in group E (continuous centrifugation during pregnancy and nursing). On P21 the largest decrease in cerebellar mass was recorded in group D (exposure from P6-P21); cerebellar mass of males was decreased by 17% and that of females by 12.7%. Continuous exposure to hypergravity during pregnancy and nursing resulted in decreases in cerebellar mass of 4.5% in males and a 12.7% in females. The third most significant change was observed in the offspring of dams exposed to hypergravity during the second week of pregnancy (group A); male cerebellar mass was decreased by 4% and female cerebellar mass by 2.1%. ANOVA of cerebellar mass on P21 showed a major treatment effect F(4,106)=6.38, p<0.0001.
Table 2.
| EXPERIMENTAL GROUP | CEREBELLAR MASS OF MALES (GRAMS ±S.E.M.) | SIGNIFICANCE LEVEL | CEREBELLAR MASS OF FEMALES(GRAMS± S.E.M.) | SIGNIFICANCE LEVEL |
|---|---|---|---|---|
| P12 | ||||
| A | 0.105±0.004 | 0.108±0.003 | ||
| B | 0.095±0.008 | 0.092±0.006 | ||
| C | 0.080±0.006 | 0.095± | ||
| D | ||||
| E | 0.094±0.006 | 0.092±0.005 | ||
| F | 0.112±0.006 | 0.115±0.002 | ||
| P21 | ||||
| A | 0.192±0.010 | 0.185±0.006 | ||
| B | 0.196±0.003 | 0.190±0.001 | ||
| C | 0.197±0.005 | 0.188±0.007 | ||
| D | 0.166±0.004 | p<0.0001 | 0.165±0.005 | p<0.0001 |
| E | 0.191±0.002 | p=0.025 | 0.177±0.004 | p=0.025 |
| F | 0.200±0.002 | 0.189±0.003 |
Effect of exposure to 1.65G during different developmental periods on cerebellar mass on P12 and P21 (mean ± S.E.M.). Cerebellar mass on P12 was most affected in male neonates exposed immediately after birth (group C). On P21, cerebellar mass was most affected in male and female neonates exposed to hypergravity during the 2nd and 3rd week of nursing (group D), with males being more affected (p<0.0001).
Maternal food consumption.
Decreased food consumption was observed in all HG dams during the first nine days following the onset of centrifugation (Ladd et al., submitted). Dams in all HG groups failed to fully recover from this initial loss of body mass. Consequently, total food consumption was decreased in all HG dams, but the magnitude of the decrease differed depending on the period of exposure (Table 3). ANOVA showed a significant effect of exposure to hypergravity (p<0.0001) and period of exposure (p<0.0001). The least amount of food consumed by dams over the course of the experiment (G3-P21) was observed in group E (exposed to 1.65G during both pregnancy and nursing) where the consumption was decreased by 23.7% compared to group F (SC dams). Food consumption was also substantially decreased in group A (dams exposed to 1.65G during the 2nd week of pregnancy only) and in group D (dams exposed to 1.65G during the 2nd and 3rd weeks of nursing). Food consumption was decreased by 18.6% in group E, by 13.9% in group A, and by 12.4% in group D. It is important to point out that all dams were matched with respect to body mass at the beginning of the experiment. Thus the difference in food consumption was not due to differences in body mass.
Table 3.
| EXPERIMENTAL GROUP | TOTAL MATERNAL (G3- P21) FOOD CONSUMED IN GRAMS ± STD. ERR. | SIGNIFICANCE LEVEL |
|---|---|---|
| A | 38.38±2.70 | p<0.0001 |
| B | 41.32±0.81 | p=0.0211 |
| C | 40.32±0.78 | p=0.0071 |
| D | 38.70±1.24 | p=0.0002 |
| E | 36.68±1.46 | P<0.0001 |
| F | 44.4±1.57 |
Effect of exposure to 1.65G during different developmental periods on total maternal food consumption from G3-P21 (mean ± S.E.M.). Food consumption was reduced in dams exposed to 1.65G during both pregnancy and nursing (group E), during the second week of pregnancy (group A) and during the 2nd and 3rd week of nursing (group D).
4. Discussion
The phases of fetal and neonatal development together constitute a period of heightened vulnerability of the CNS to environmental perturbations. During this time the developing CNS is undergoing genetically programmed cell birth, proliferation, migration, synaptogenesis and cell apoptosis. Disturbances in any of these processes can affect CNS structure and function. The current study focuses upon the effect of altered gravity on the developing CNS. Since both astronauts (Newberg, 1994) and adult animals exposed to altered gravity (Katafuchi et al., 1995) experience disturbances in motor coordination, we hypothesized that exposure to hypergravity during the period of heightened vulnerability of prenatal and postnatal development would magnify the changes observed in adults. The results presented here support our hypothesis showing that both prenatal and early postnatal exposure to hypergravity affects neonatal growth and CNS development. Our data also suggest an existence of discrete temporary windows of vulnerability of the developing organism to altered gravity. These windows of vulnerability should be considered in planning future human space ventures, since the same developmental sequences occur in humans and rats, although the timing relative to birth is different. In humans, cerebellar developmental processes during the third trimester of pregnancy correspond to the changes occurring in rats during the first 10 days after birth (Dobbing and Sands, 1979); this period is referred to as “third trimester equivalent.”
Prenatal exposure to hypergravity affects intrauterine growth, as suggested by a decrease in the number of pups in a litter and the average neonatal mass at birth, with the exposure during the 3rd week of pregnancy being especially critical. On the other hand, the neonatal mortality was equally affected by the exposure to hypergravity throughout the pregnancy. One unexpected finding was that the highest mortality occurred in neonates transferred onto the centrifuge immediately after birth. Particularly surprising was the observation that the highest mortality occurred between the 3rd (P3) and 6th (P6) day. The maximal mortality coincided with the so-called critical developmental period identified in rats as between P4 and P9 (Pierce et al., 1999). Furthermore, more than half of the pups transferred to hypergravity at birth were cannibalized, although it is unclear at this point whether HG dams cannibalized living neonates or whether the neonates died of other causes and were subsequently cannibalized. It is possible that dams challenged by the nutritional and energy demands of nursing consumed the weakest pups. Apparently energy demands during lactation increase 16-fold over gestational demands (Tran and Kelly, 2003).
Neonatal growth was more strongly affected by postnatal than prenatal exposure to hypergravity. Interestingly, chronic exposure to centrifugation during both pregnancy and nursing had no effect on neonatal body mass on P21, suggesting a gradual adaptation that allowed normal postnatal development.
The sequential appearance of developmental markers was also affected by the period of exposure to hypergravity. Righting time was maximally increased in male neonates exposed to hypergravity immediately after birth; in female pups righting time was equally increased by prenatal and postnatal exposure. Prenatal exposure to hypergravity increased the righting time significantly more in male than in female pups. The startle response was delayed both in male and in female pups exposed to 1.65G. However, the delay was different depending on the period of exposure. Eye opening was slightly delayed in male neonates in all experimental groups. On P14 it was most affected in male neonates exposed to hypergravity during the first postnatal week; the most strongly affected female neonates were those exposed to hypergravity during the last week of pregnancy, but the data was not statistically significant. Others (Bouet et al., 2004b) observed a similar delay in growth and increased righting time. However, advanced eye opening was observed in rats developing in 2G but transferred to 1G on P5 or P10. It is possible that transition from 2G to 1G stimulates eye opening.
Could some of these effects result from lower food consumption by hypergravity-exposed dams? Data presented here indicate that the impairment of motor functions appears to coincide with lower food consumption and lower cerebellar mass. However the relationship is not statistically significant, supporting the hypothesis that a complexity of factors affects rotarod performance. It has been observed that chronic maternal undernutrition in rat such as under food restriction conditions results in a significant loss of body mass throughout pregnancy and results in lower neonatal body mass at birth, but without the change in litter size (Woodall et al., 1996). Our dams were not restricted in food and they experienced only transient loss in body mass, but both the litter size and the neonatal mass were affected. The lack of similar changes in RC group seems to argue further against a general and nonspecific stress of centrifugation and suggest that there may be a specific graviperception triggering the response in hypergravity-exposed animals.
Motor coordination was impaired in male and female neonates in all HG groups except for those exposed to hypergravity during the third week of pregnancy, and the effect was sex-selective. These results complement data on motor dysfunction from other behavioral tests in hypergravity-exposed mammals. Inhibition of motor function by perinatal hypergravity exposure (2.5G) was seen on P28 in swimming and air-righting tests in hamsters (Sondag et al., 1997) and in exploratory tests in juvenile rats exposed to hypergravity (1.8G; Thullier et al., 2002). Motor functions and equilibrium also were significantly inhibited in Wistar-SPF rats flown on board Cosmos biosatellites (Aizikov and Markin, 1981). Impairments in motor functions thus occur in both hypo- and hypergravity, a finding that is not surprising in light of the known influence of Earth’s gravity on the formation and development of motor functions (Laputin and Kadenyuk, 1999). While the changes in gravity affected motor functions in both developing and adult organisms and may be related to altered vestibulocerebellar changes, the effect of hypergravity on motor functions appeared to be especially significant in the developing organism.
While both male and female pups were most affected by exposure during the 2ndweek of pregnancy and 2nd and 3rd weeks of lactation, the most pronounced effect was observed in male pups exposed to 1.65G during the 2nd week of pregnancy, among which the latency before falling was five times shorter than in the SC group. Cerebellar mass was most significantly affected by exposure to hypergravity during the brain growth spurt on P4-P9 with the effect being more pronounced in males. Neonates exposed to 1.65 G during fetal development attained normal cerebellar mass on P21. While there appears to be some correlation between decreased cerebellar mass on P21 and reduced performance on the rotarod test in rat neonates during individual periods of exposure, that correlation is far from perfect. Thus, other factors, such as cerebellar cell number (Sajdel-Sulkowska et al., 2005), cellular rearrangements and consequently cerebellar circuitry must be considered (Andersen, 2003). Developmental markers, including the maturation of spinal motor neurons (Brocard et al., 2003) and the maturation of the vestibular system (Gaboyard et al., 2003) are also affected by hypergravity exposure and are likely to contribute to impaired performance on the rotarod.
Thus, we can point out two periods of sensitivity of cerebellum to hypergravity exposure: the 2nd week of pregnancy, corresponding to early stages of human pregnancy (1st/2nd) and the 2nd/3rd postnatal weeks corresponding to the third trimester of pregnancy and early postpartum in humans. Exposure to hypergravity during the 2nd week of the rat’s pregnancy coincides with the period of Purkinje cell birth on G13/G14, and exposure during the 2nd and the 3rd postnatal week coincides with the peak in granule cell neurogenesis around P13 (Altman, 1996). Thus, the impairment of motor functions corresponds to specific cellular events in the cerebellum. In other brain regions that develop primarily during the prenatal period, the window(s) of heightened vulnerability to hypergravity (or other environmental perturbations) may be different.
The effect of hypergravity on the developing CNS has been observed in fish where exposure to 3G affects cell number of the visual Nucleus isthmi (Anken et al., 2002) and the size and asymmetry of inner ear otoliths as well as swimming behavior (Anken et al., 1998). Furthermore, developmental stage-specific sensitivity of vestibuloocular reflex to hypergravity has been observed in amphibians (Sebastian et al., 1998); it has been suggested that the periods of sensitivity may be related to neurogenensis and synaptogenesis.
In conclusion:
The effects of perinatal exposure to hypergravity on neonatal growth and CNS development are strongly dependent on the specific developmental stage at which the exposure occurs. Prenatal hypergravity exposure affects intrauterine growth, while postnatal exposure impedes neurodevelopment, motor coordination, and tends to limit cerebellar mass. The magnitude of the hypergravity effect on the developing CNS is further determined by discrete windows of vulnerability. The 2nd week of pregnancy appears to be more critical than the third week of pregnancy, and the period P6-P21 appears to be more critical than the immediate postpartum period with respect to maturation of cerebellar structure and function. In rats, this period overlaps with a brain growth spurt (P4-P9) and corresponds to the 3rd trimester of human pregnancy. There appear to be two windows of vulnerability to hypergravity-induced changes in motor functions: one prenatal and one postnatal. Early prenatal exposure to hypergravity is especially detrimental to motor development in male offspring suggesting sexually dimorphic vulnerability to the environmental perturbations. The sexually dimorphic nature of the effect of altered gravity on the developing CNS should be taken into consideration while planning future human space exploration and habitation.
Acknowledgements
This study was supported by NIEHS grant 11946. We thank C. Cleveland, J. Light, V.A. Sulkowski and Z.L. Sulkowski for their help in carrying out daily tasks associated with the centrifugation. We would like thank Tianna Shaw, Manager, Facilities Utilization Office Life Sciences Division NASA Ames Research Center for the use of the facility. We thank Stephen Voels for coordinating experimental activities and Dr. Geoffrey Bush for his excellent help with the experimental protocol.
References
- Aizikov GS, Markin AS. Postures, movements and equilibrium functions of rats after flights on biosatellites. Kosm. Biol. Aviakosm. Med. 1981;15:33–38. [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of cerebellum. CRC Press, Inc; Boca Raton, Fl: 1997. [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Behavior. Res. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Anken RH, Kappel T, Rahmann H. Morphometry of fish inner ear otoliths after development at 3g hypergravity. Acta Otolaryngol. 1998;118:534–539. doi: 10.1080/00016489850154685. [DOI] [PubMed] [Google Scholar]
- Anken RH, Werner K, Rahmann H. Effects of hypergravity on the development of cell number and asymmetry in fish brain nuclei. Adv. Space Res. 2002;30:849–853. doi: 10.1016/s0273-1177(01)00644-5. [DOI] [PubMed] [Google Scholar]
- Auvray N, Caston J, Reber A, Stelz T. Role of the cerebellum in the ontogenensis of the equilibrium behavior in the young rat: a behavioral study. Brain Res. 1989;505:291–301. doi: 10.1016/0006-8993(89)91455-8. [DOI] [PubMed] [Google Scholar]
- Bouet V, Ijkema-Paassen J, Wubbels R, Gramsbergen A. Development of the locomotor system in 2G exposed rats. J. Gravit. Physiol. 2004a;11:165–166. [PubMed] [Google Scholar]
- Bouet V, Wubbels RJ, de Jong HA, Gramsbergen A. Behavioural consequences of hypergravity in developing rats. Brain Res. Dev. Brain Res. 2004b;153:69–78. doi: 10.1016/j.devbrainres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Brocard F, Clarac F, Vinay L. Gravity influences the development of inputs from the brain to lumbar motorneurons in the rat. Neuroreport. 2003;14:1697–1700. doi: 10.1097/00001756-200309150-00008. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Fritzsch B. The development of vestibular connections in rat embryos in microgravity. J. Grav. Physiol. 1997;4:59–62. [PubMed] [Google Scholar]
- Caston J, Lalonde R, Delhaye-Bouchaud N, Mariani J. The cerebellum and the postural sensimotor learning in mice and rats. Behav. Brain res. 1998;95:17–22. doi: 10.1016/s0166-4328(97)00205-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Develop. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Gaboyard S, Sans A, Lehouelleur J. Differential impact of hypergravity on maturating innervations in vestibular epithelia during rat development. Brain Res. Dev. Brain res. 2003;143:15–23. doi: 10.1016/s0165-3806(03)00069-5. [DOI] [PubMed] [Google Scholar]
- Gimenez y Ribotta M, Sandillon F, Privat A. Influence of hypergravity on the development of monoaminergic system in the rat spinal cord. Brain Res. Dev. Brain Res. 1998;111:147–157. doi: 10.1016/s0165-3806(98)00132-1. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, paiva M. Ethanol-induced alterations in neurotrophin expression in developing cerebellum: relationship to periods of temporal susceptibility. Alcohol. Clin. Exp. Res. 1999;23:1637–1642. [PubMed] [Google Scholar]
- Katafuchi T, Hori T, Oomura Y, Koizumi K. Cerebellar affernts to neuroendocrine cell: implications for adaptive responses to stimulated weightlessness. Endocrine J. 1995;42:729–737. doi: 10.1507/endocrj.42.729. [DOI] [PubMed] [Google Scholar]
- Ladd B, Nguon K, Sajdel-Sulkowska EM. Exposure to hypergravity affects pregnant rat dams and pregnancy outcome. Adv. Space Res. 2005 (accepted) [Google Scholar]
- Laputin AN, Kadenyuk LK. Change-over coordination structure of astronauts’ voluntary movements during orbital flight conditions. J. Gravit. Physiol. 1999;6:121–122. [PubMed] [Google Scholar]
- Layne CS, McDonald PV, Bloomberg JJ. Neuromascular activation patterns during treadmill walking after space flight. Exp. Brain Res. 1997;113:104–116. doi: 10.1007/BF02454146. [DOI] [PubMed] [Google Scholar]
- Newberg AB. Changes in the central nervous system and their clinical correlates during long-term spaceflight. Aviat. Space Environ. Med. 1994;65:562–572. [PubMed] [Google Scholar]
- Nguon K, Li G-H, Sajdel-Sulkowska EM. CNS development under altered gravity: Cerebellar glial and neuronal protein expression in rat neonates exposed to hypergravity. Adv. Space Res. 2004;33:1375–1380. doi: 10.1016/j.asr.2003.06.016. [DOI] [PubMed] [Google Scholar]
- Nguon K, Baxter MG, Saidel-Sulkowska EM. Perinatal exposure to polychlorinated biphenyls differentially affects cerebellar development and motor functions in male and female neonates. Cerebellum. 2005a;4:112–122. doi: 10.1080/14734220510007860. [DOI] [PubMed] [Google Scholar]
- Nguon K, Ladd B, Sajdel-Sulkowska EM. Development of motor coordination and cerebellar structure in male and female rat neonates exposed to hypergravity. Adv. Space Res. 2005b doi: 10.1016/j.asr.2003.06.016. (accepted) [DOI] [PubMed] [Google Scholar]
- Pierce DR, Willams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcohol. Clin. Exp. Res. 1999;23:1650–1659. [PubMed] [Google Scholar]
- Reschke MF, Bloomberg JJ, Harm DL, et al. Posture, locomotion, spatial orientation, and motion sickness as function of space flight. Brain Res. Brain Res. Rev. 1998;28:102–107. doi: 10.1016/s0165-0173(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Li GH, Ronca AE, et al. Effects of hypergravity exposure on the developing central nervous system: possible involvement of thyroid hormone. Exp. Biol. Med. 2001;226:790–798. doi: 10.1177/153537020222600812. [DOI] [PubMed] [Google Scholar]
- SajdelSulkowska EM, Nguon K, Rosen G, Baxter MG. Purkinje cell loss and motor impairment in rats developing under hypergravity. NeuroReport. 2005;16:2037–2040. doi: 10.1097/00001756-200512190-00014. [DOI] [PubMed] [Google Scholar]
- Savel’ev SV, Serova LV, Besova NV, et al. Effect of weightlessness on rat’s endocrine system development. Aviakosm. Ekolog. Med. 1998;32:31–36. [PubMed] [Google Scholar]
- Schmitz C, Dafotakis M, Heinsen H, et al. Use of cryostat sections from snap-frozen nervous tissue for combining stereological estimates with histological, cellular and molecular analyses on adjacent sections. J. Chem. Neuroanat. 2000;20:21–29. doi: 10.1016/s0891-0618(00)00075-2. [DOI] [PubMed] [Google Scholar]
- Sebastian CE, Pfau K, Horn ER. An age-dependent sensitivity of the roll-induced vestibuloocular reflex to hypergravity exposure of several days in an amphibian (Xenopus laevis) Acta Astronaut. 1998;42:419–430. doi: 10.1016/s0094-5765(98)00136-2. [DOI] [PubMed] [Google Scholar]
- Serova LV, Denisova LA, Pustynnikova AM. Comparative analysis of hypo- and hypergravity effects on prenatal development of mammals. Physiologist. 1985;28:s5–s8. [PubMed] [Google Scholar]
- Sondag HN, de Jong HA, Oosterveld WJ. Altered behavior in hamsters conceived and born in hypergravity. Brain res. Bull. 1997;43:289–294. doi: 10.1016/s0361-9230(97)00008-7. [DOI] [PubMed] [Google Scholar]
- Sulkowski GM, Li G-H, Sajdel-Sulkowska EM. CNS development under altered gravity: CD15, NCAM and GFAP expression in rat neonates exposed to hypergravity. Adv. Space Res. 2004;33:1423–1430. doi: 10.1016/j.asr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Tafforin C. Relationship between orientation, movement and posture in weightlessness: preliminary ethological observations. Acta Astronaut. 1990;21:271–280. doi: 10.1016/0094-5765(90)90050-u. [DOI] [PubMed] [Google Scholar]
- Thullier F, Hayzoun K, Dubois M, Lestienne F, Lalonde R. Exploration and motor activity in juvenile and adult rats exposed to hypergravity at 1.8G during development: a preliminary report. Physiol. Behav. 2002;76:617–622. doi: 10.1016/s0031-9384(02)00766-7. [DOI] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol. Teratol. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Holmes MJ, Jian BJ. Plastic changes in processing of graviceptive signals during spaceflight potentially contribute to postflight orthostaticin tolerance. J. Vest. Res. 2003;13:395–404. [PubMed] [Google Scholar]