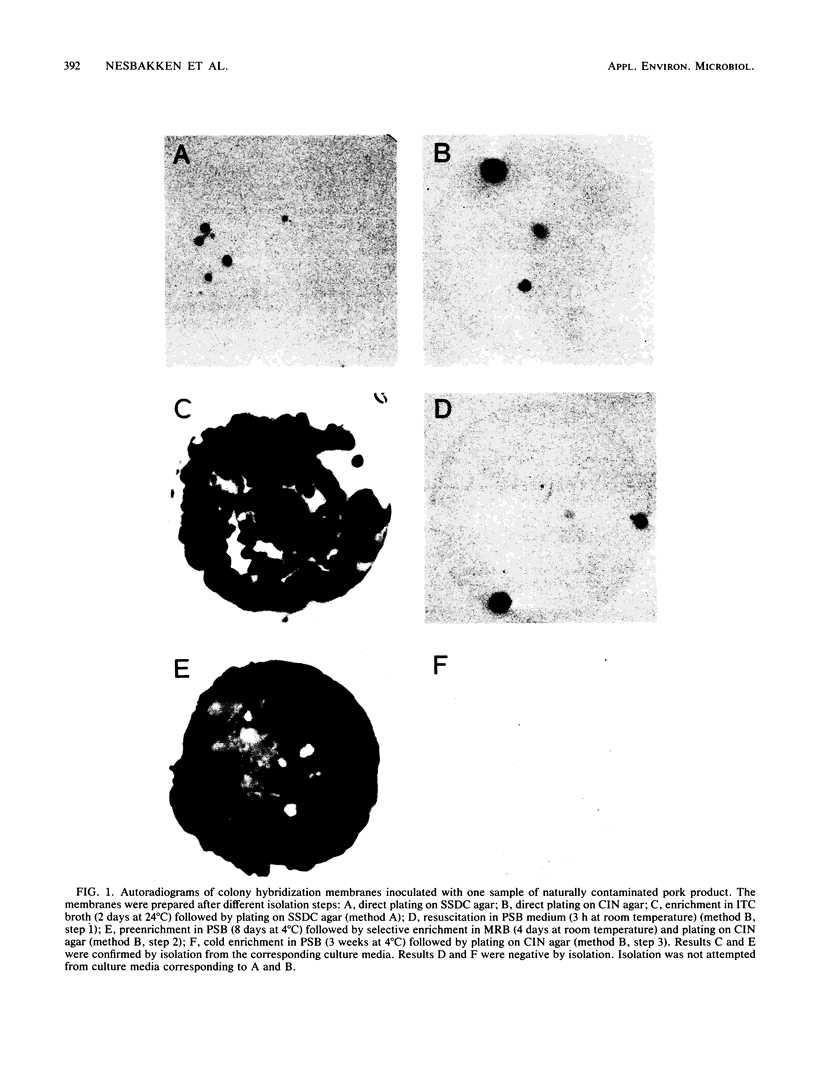
Abstract
We compared a DNA-DNA hybridization assay, using a synthetically produced oligonucleotide probe, and two conventional isolation procedures (methods A and B) with regard to their relative efficiency in detecting Yersinia enterocolitica O:3 in naturally contaminated pork products. Method A was as described by Wauters et al. (Appl. Environ. Microbiol. 54:851-854, 1988). Method B has been recommended by the Nordic Committee on Food Analysis (method no. 117, 1987). The genetic probe was used in a colony hybridization assay to detect virulent yersiniae at each of the isolation steps with composed methods A and B. A total of 50 samples of raw pork products obtained from 13 meat-processing factories in Norway were examined. Y. enterocolitica serogroup O:3, biovar 4, was isolated from altogether 9 (18.0%) of the samples by using the two isolation procedures. In contrast, colony hybridization using the genetic probe indicated that 30 (60.0%) of the samples contained virulent yersiniae. All samples which were positive on cultivation were also positive by hybridization. The results indicate that the occurrence of pathogenic Y. enterocolitica in Norwegian pork products is substantially higher than previously demonstrated and, therefore, reinforce our suggestion that pork products represent an important potential source of human infection in Norway. The results also indicate that the use of conventional isolation procedures may lead to considerable underestimation of pathogenic Y. enterocolitica in pork products.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gemski P., Sodd M. A., Neill R. J., Seguin M. C., Williams J. E. Cloning and use of Vwa plasmid DNA as gene probes for virulent Yersiniae. Contrib Microbiol Immunol. 1987;9:296–303. [PubMed] [Google Scholar]
- Hill W. E., Payne W. L., Aulisio C. C. Detection and enumeration of virulent Yersinia enterocolitica in food by DNA colony hybridization. Appl Environ Microbiol. 1983 Sep;46(3):636–641. doi: 10.1128/aem.46.3.636-641.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagow J., Hill W. E. Enumeration by DNA colony hybridization of virulent Yersinia enterocolitica colonies in artificially contaminated food. Appl Environ Microbiol. 1986 Feb;51(2):441–443. doi: 10.1128/aem.51.2.441-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Dommarsnes K., Skurnik M., Hornes E. A synthetic oligonucleotide probe and a cloned polynucleotide probe based on the yopA gene for detection and enumeration of virulent Yersinia enterocolitica. Appl Environ Microbiol. 1990 Jan;56(1):17–23. doi: 10.1128/aem.56.1.17-23.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Nesbakken T., Aleksic S., Mollaret H. H. Comparison of restriction endonuclease analysis and phenotypic typing methods for differentiation of Yersinia enterocolitica isolates. J Clin Microbiol. 1990 Jun;28(6):1125–1131. doi: 10.1128/jcm.28.6.1125-1131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen J. Rapid identification of gram-negative rods using a three-tube method combined with a dichotomic key. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):525–533. doi: 10.1111/j.1699-0463.1975.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Miliotis M. D., Galen J. E., Kaper J. B., Morris J. G., Jr Development and testing of a synthetic oligonucleotide probe for the detection of pathogenic Yersinia strains. J Clin Microbiol. 1989 Jul;27(7):1667–1670. doi: 10.1128/jcm.27.7.1667-1670.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaret H. H., Bercovier H., Alonso J. M. Summary of the data received at the WHO Reference Center for Yersinia enterocolitica. Contrib Microbiol Immunol. 1979;5:174–184. [PubMed] [Google Scholar]
- Nesbakken T. Comparison of sampling and isolation procedures for recovery of Yersinia enterocolitica serotype O:3 from the oral cavity of slaughter pigs. Acta Vet Scand. 1985;26(1):127–135. doi: 10.1186/BF03546570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbakken T. Enumeration of Yersinia enterocolitica O:3 from the porcine oral cavity, and its occurrence on cut surfaces of pig carcasses and the environment in a slaughterhouse. Int J Food Microbiol. 1988 Jun;6(4):287–293. doi: 10.1016/0168-1605(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Nesbakken T., Kapperud G., Sørum H., Dommarsnes K. Structural variability of 40-50 Mdal virulence plasmids from Yersinia enterocolitica. Geographical and ecological distribution of plasmid variants. Acta Pathol Microbiol Immunol Scand B. 1987 Jun;95(3):167–173. doi: 10.1111/j.1699-0463.1987.tb03107.x. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Miliotis M. D., Cianciosi S., Miller V. L., Falkow S., Morris J. G., Jr Evaluation of DNA colony hybridization and other techniques for detection of virulence in Yersinia species. J Clin Microbiol. 1989 Apr;27(4):644–650. doi: 10.1128/jcm.27.4.644-650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A. Development of a two-step enrichment procedure for recovery of Yersinia enterocolitica from food. Appl Environ Microbiol. 1982 Jan;43(1):14–27. doi: 10.1128/aem.43.1.14-27.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol Microbiol. 1989 Apr;3(4):517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Vandepitte J., Wauters G., Martin S. M., Goossens V., De Mol P., Van Noyen R., Thiers G. Yersinia enterocolitica infections and pork: the missing link. Lancet. 1987 May 16;1(8542):1129–1132. doi: 10.1016/s0140-6736(87)91683-7. [DOI] [PubMed] [Google Scholar]
- Wauters G., Goossens V., Janssens M., Vandepitte J. New enrichment method for isolation of pathogenic Yersinia enterocolitica serogroup O:3 from pork. Appl Environ Microbiol. 1988 Apr;54(4):851–854. doi: 10.1128/aem.54.4.851-854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauters G., Kandolo K., Janssens M. Revised biogrouping scheme of Yersinia enterocolitica. Contrib Microbiol Immunol. 1987;9:14–21. [PubMed] [Google Scholar]