Abstract
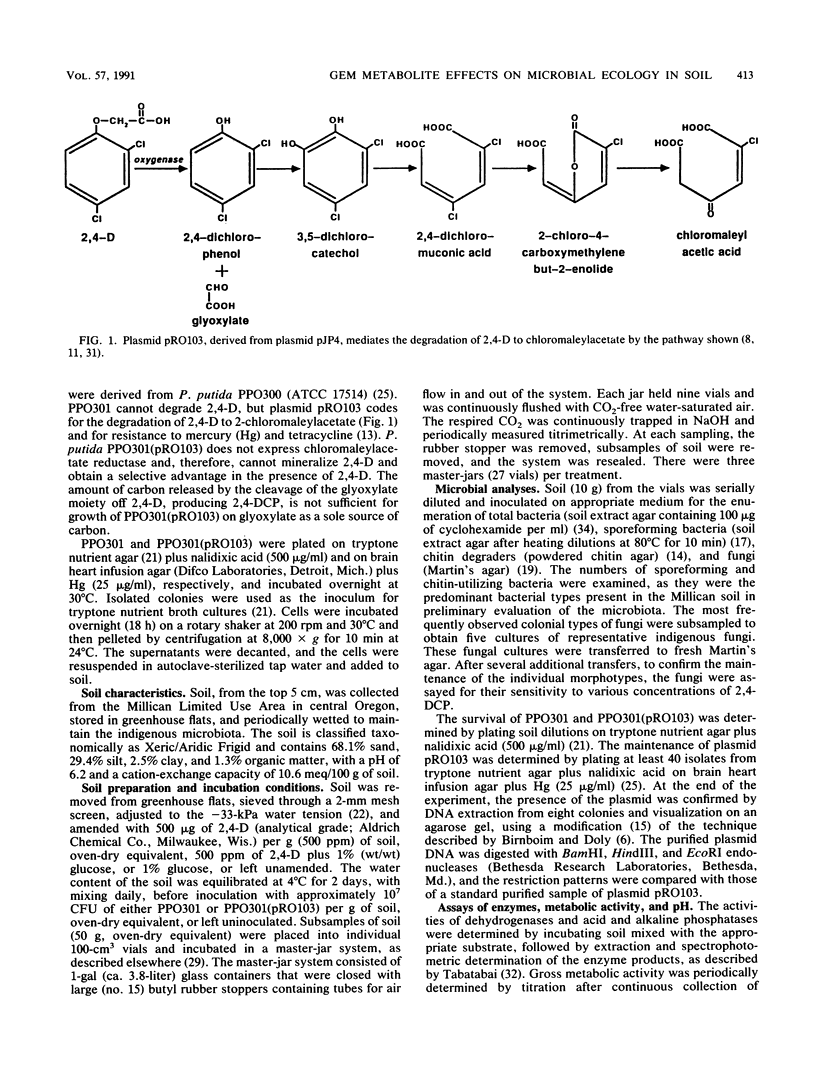
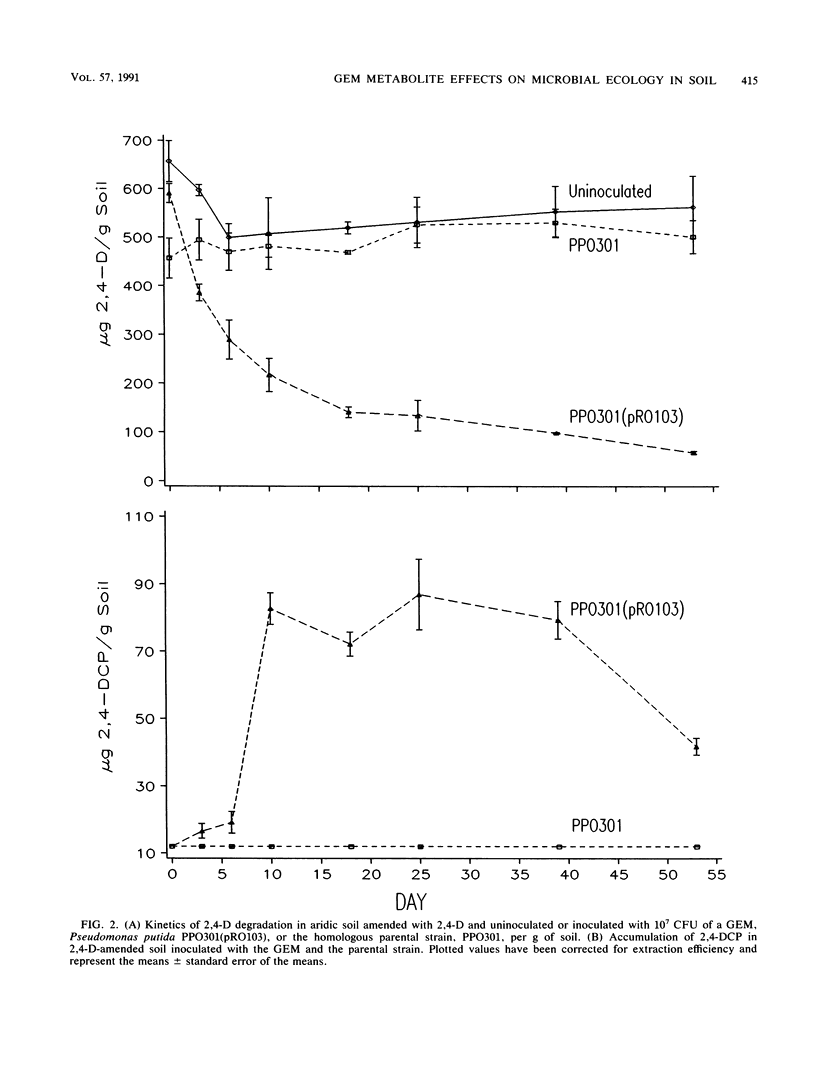
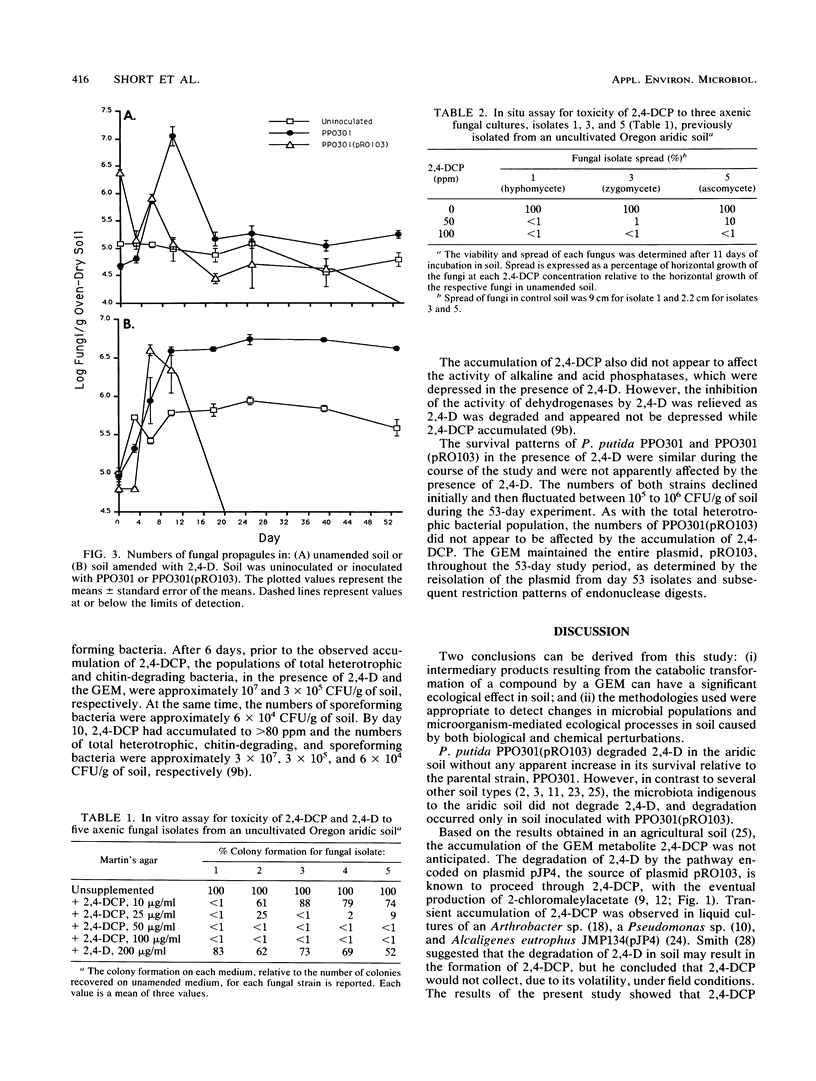
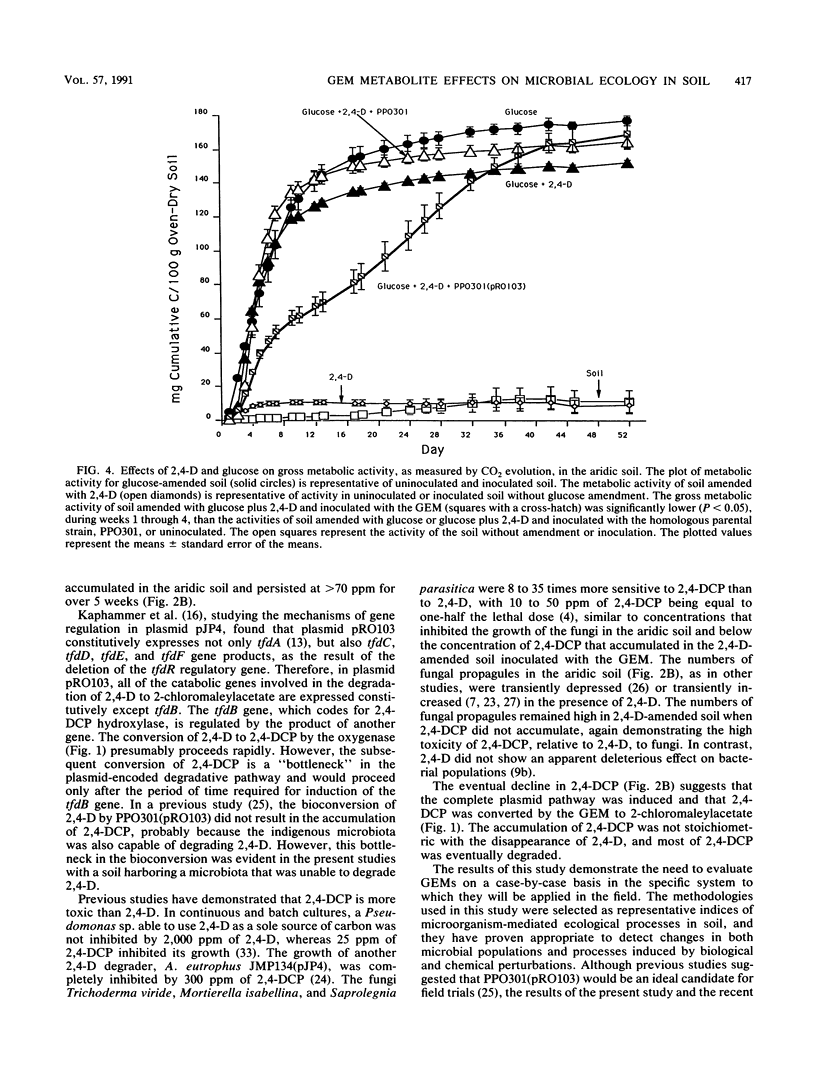
A genetically engineered microorganism, Pseudomonas putida PPO301(pRO103), and the plasmidless parent strain, PPO301, were added at approximately 107 CFU/g of soil amended with 500 ppm of 2,4-dichlorophenoxyacetate (2,4-D) (500 μg/g). The degradation of 2,4-D and the accumulation of a single metabolite, identified by gas chromatography-mass spectrophotometry as 2,4-dichlorophenol (2,4-DCP), occurred only in soil inoculated with PPO301(pRO103), wherein 2,4-DCP accumulated to >70 ppm for 5 weeks and the concentration of 2,4-D was reduced to <100 ppm. Coincident with the accumulation of 2,4-DCP was a >400-fold decline in the numbers of fungal propagules and a marked reduction in the rate of CO2 evolution, whereas 2,4-D did not depress either fungal propagules or respiration of the soil microbiota. 2,4-DCP did not appear to depress the numbers of total heterotrophic, sporeforming, or chitin-utilizing bacteria. In vitro and in situ assays conducted with 2,4-DCP and fungal isolates from the soil demonstrated that 2,4-DCP was toxic to fungal propagules at concentrations below those detected in the soil.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbuchner J., Schmid K., Schmitt R. Tn1721-encoded tetracycline resistance: mapping of structural and regulatory genes mediating resistance. J Bacteriol. 1983 Jan;153(1):116–123. doi: 10.1128/jb.153.1.116-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Schulke J. W., Frazier L. M., Seidler R. J. Characterization of aquatic bacteria and cloning of genes specifying partial degradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1985 May;49(5):1237–1245. doi: 10.1128/aem.49.5.1237-1245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breazeale F. W., Camper N. D. Bacterial, fungal, and actinomycete populations in soils receiving repeated applications of 2,4-dichlorophenoxyacetic acid and trifluralin. Appl Microbiol. 1970 Feb;19(2):379–380. doi: 10.1128/am.19.2.379-380.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Genetic and physical map of the 2,4-dichlorophenoxyacetic acid-degradative plasmid pJP4. J Bacteriol. 1985 Jan;161(1):466–468. doi: 10.1128/jb.161.1.466-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Weightman A. J., Knackmuss H. J., Timmis K. N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J Bacteriol. 1985 Jan;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S. Nucleotide homology and organization of chlorocatechol oxidation genes of plasmids pJP4 and pAC27. Mol Gen Genet. 1988 Jan;211(1):113–120. doi: 10.1007/BF00338401. [DOI] [PubMed] [Google Scholar]
- Harker A. R., Olsen R. H., Seidler R. J. Phenoxyacetic acid degradation by the 2,4-dichlorophenoxyacetic acid (TFD) pathway of plasmid pJP4: mapping and characterization of the TFD regulatory gene, tfdR. J Bacteriol. 1989 Jan;171(1):314–320. doi: 10.1128/jb.171.1.314-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. C., Lockwood J. L. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol. 1975 Mar;29(3):422–426. doi: 10.1128/am.29.3.422-426.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphammer B., Kukor J. J., Olsen R. H. Regulation of tfdCDEF by tfdR of the 2,4-dichlorophenoxyacetic acid degradation plasmid pJP4. J Bacteriol. 1990 May;172(5):2280–2286. doi: 10.1128/jb.172.5.2280-2286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M. A., Roberts R. N., Alexander M. Formation of 2,4-dichlorophenol and 2,4-dichloroanisole from 2,4-dichlorophen-oxyacetate by Arthrobacter sp. Can J Microbiol. 1967 Jun;13(6):691–699. doi: 10.1139/m67-091. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E. Identification of 2,4-dichloroanisole and 2,4-dichlorophenol as soil degradation products of ring-labelled [14C]2,4-D. Bull Environ Contam Toxicol. 1985 Feb;34(2):150–157. doi: 10.1007/BF01609717. [DOI] [PubMed] [Google Scholar]
- Stotzky G. Replica plating technique for studying microbial interactions in soil. Can J Microbiol. 1965 Aug;11(4):629–636. doi: 10.1139/m65-084. [DOI] [PubMed] [Google Scholar]
- Streber W. R., Timmis K. N., Zenk M. H. Analysis, cloning, and high-level expression of 2,4-dichlorophenoxyacetate monooxygenase gene tfdA of Alcaligenes eutrophus JMP134. J Bacteriol. 1987 Jul;169(7):2950–2955. doi: 10.1128/jb.169.7.2950-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. E., Finn R. K. Growth rates of a pseudomonad on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol. Appl Microbiol. 1974 Aug;28(2):181–184. doi: 10.1128/am.28.2.181-184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



