Abstract
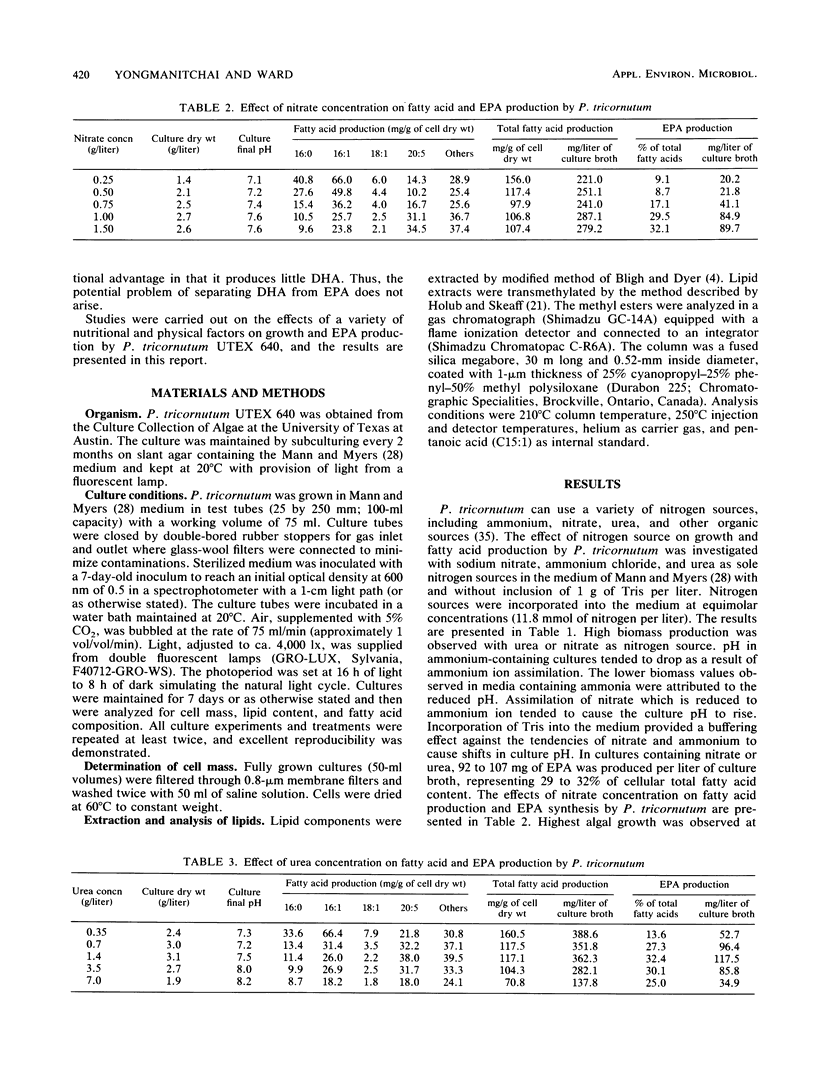
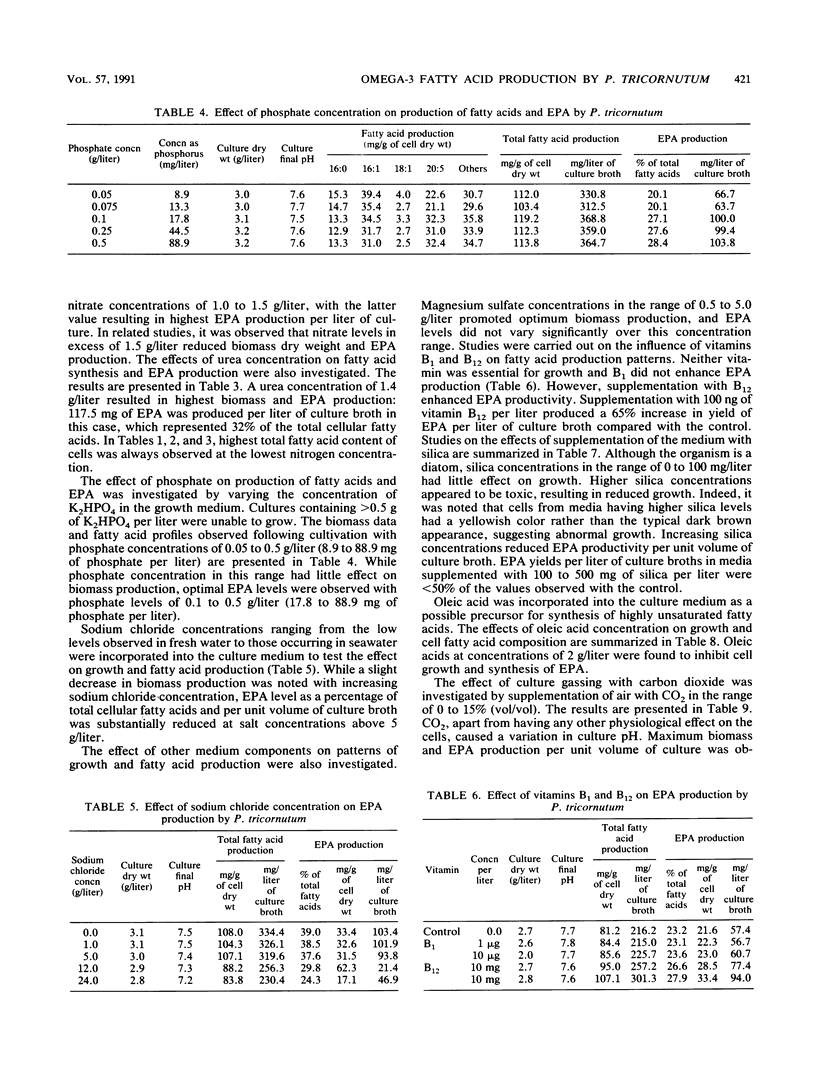
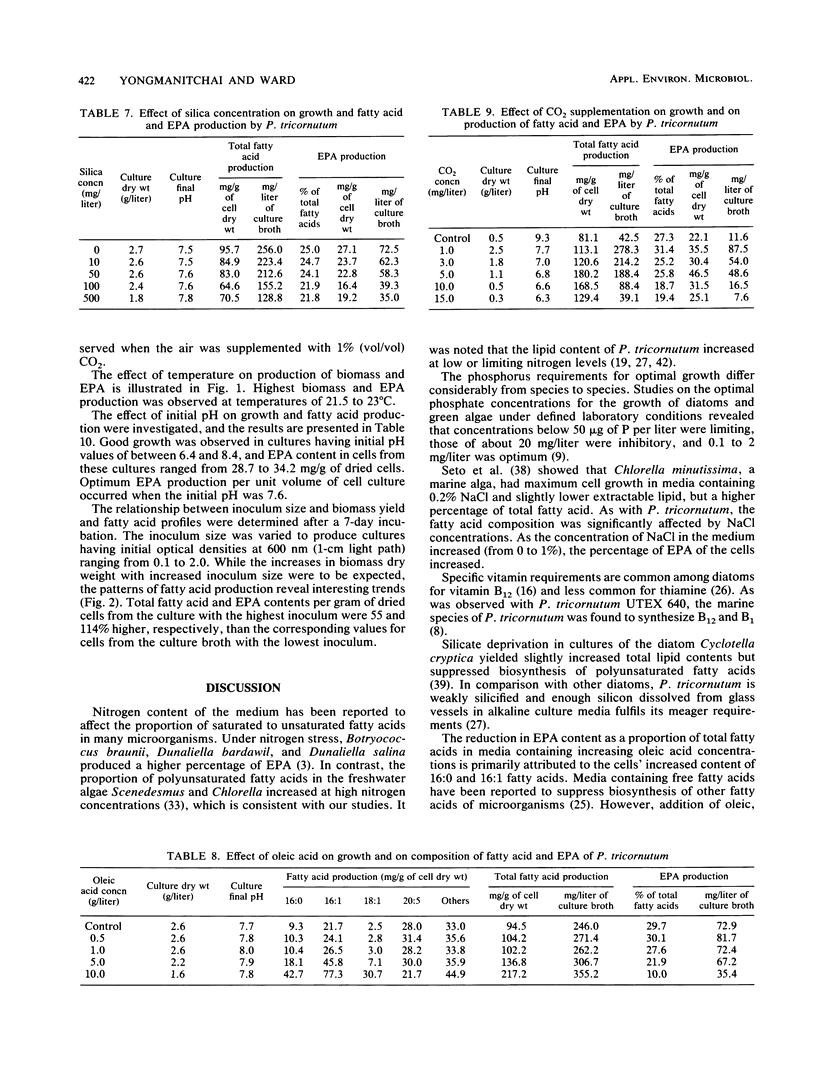
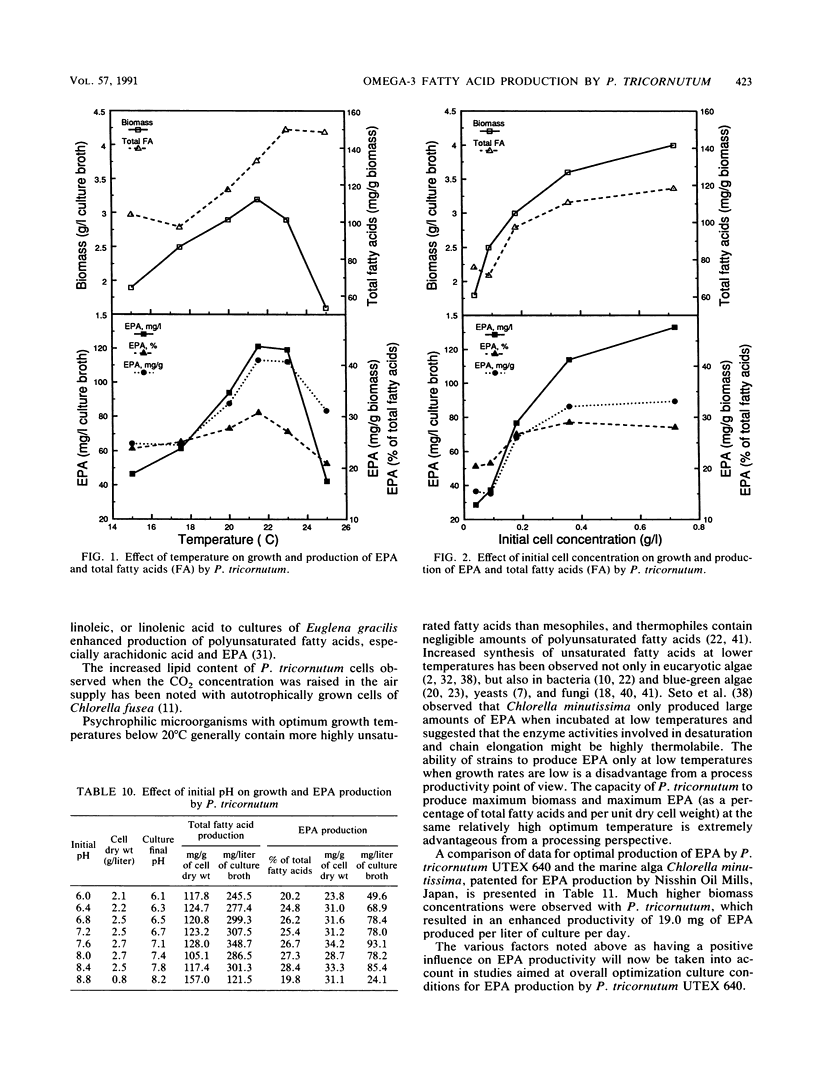
Detailed studies were carried out on the effects of nitrogen source, phosphate, sodium chloride, growth factors, precursors, CO2, temperature, initial pH, and inoculum size on biomass and eicosapentaenoic acid (EPA) production by Phaeodactylum tricornutum. The EPA content of total fatty acids increased with increasing concentrations of nitrate and urea. Sodium chloride was not required for growth or EPA production. While vitamins B1 and B12 did not affect growth significantly, EPA yield was increased by 65% by B12 supplementation. Maximum EPA production occurred when the air gassing supply was supplemented with 1% CO2. Optimum culture temperature and initial pH for EPA production were 21.5 to 23 degrees C and 7.6, respectively. EPA yields of up to 133 mg/liter of culture were observed. EPA constituted up to 30 to 40% of total fatty acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Braden L. M., Carroll K. K. Dietary polyunsaturated fat in relation to mammary carcinogenesis in rats. Lipids. 1986 Apr;21(4):285–288. doi: 10.1007/BF02536414. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Rose A. H. Fatty-acid composition of Candida utilis as affected by growth temperature and dissolved-oxygen tension. J Bacteriol. 1969 Aug;99(2):371–378. doi: 10.1128/jb.99.2.371-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daron H. H. Fatty acid composition of lipid extracts of a thermophilic Bacillus species. J Bacteriol. 1970 Jan;101(1):145–151. doi: 10.1128/jb.101.1.145-151.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson L. G., Galloway R. A., Patterson G. W. Environmentally-induced changes in the Fatty acids of chlorella. Plant Physiol. 1969 Oct;44(10):1413–1416. doi: 10.1104/pp.44.10.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978 Jul 15;2(8081):117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Dyerberg J. Linolenate-derived polyunsaturated fatty acids and prevention of atherosclerosis. Nutr Rev. 1986 Apr;44(4):125–134. doi: 10.1111/j.1753-4887.1986.tb07603.x. [DOI] [PubMed] [Google Scholar]
- Holub B. J., Skeaff C. M. Nutritional regulation of cellular phosphatidylinositol. Methods Enzymol. 1987;141:234–244. doi: 10.1016/0076-6879(87)41071-9. [DOI] [PubMed] [Google Scholar]
- KATES M., HAGEN P. O. INFLUENCE OF TEMPERATURE ON FATTY ACID COMPOSITION OF PSYCHROPHILIC AND MESOPHILIC SERRATIA SPECIES. Can J Biochem. 1964 Apr;42:481–488. doi: 10.1139/o64-055. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt M. G., McMahon V. A. Effect of Growth Temperature on the Lipid Composition of Cyanidium caldarium: II. Glycolipid and Phospholipid Components. Plant Physiol. 1970 Aug;46(2):290–293. doi: 10.1104/pp.46.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIN J. C., LEWIN R. A., PHILPOTT D. E. Observations on Phaeodactylum tricornutum. J Gen Microbiol. 1958 Apr;18(2):418–426. doi: 10.1099/00221287-18-2-418. [DOI] [PubMed] [Google Scholar]
- Lees A. M., Korn E. D. Metabolism of unsaturated fatty acids in protozoa. Biochemistry. 1966 May;5(5):1475–1481. doi: 10.1021/bi00869a005. [DOI] [PubMed] [Google Scholar]
- Mortensen J. Z., Schmidt E. B., Nielsen A. H., Dyerberg J. The effect of N-6 and N-3 polyunsaturated fatty acids on hemostasis, blood lipids and blood pressure. Thromb Haemost. 1983 Aug 30;50(2):543–546. [PubMed] [Google Scholar]
- Patterson G. W. Effect of culture temperature on fatty acid composition of Chlorella sorokiniana. Lipids. 1970 Jul;5(7):597–600. doi: 10.1007/BF02531336. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Maruyama H. Effect of dietary fish oil on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 1986 Jul;46(7):3367–3370. [PubMed] [Google Scholar]
- Robinson D. R., Prickett J. D., Makoul G. T., Steinberg A. D., Colvin R. B. Dietary fish oil reduces progression of established renal disease in (NZB x NZW)F1 mice and delays renal disease in BXSB and MRL/1 strains. Arthritis Rheum. 1986 Apr;29(4):539–546. doi: 10.1002/art.1780290412. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Shinmen Y., Kawashima H., Akimoto K., Yamada H. Fungal mycelia as a novel source of eicosapentaenoic acid. Activation of enzyme(s) involved in eicosapentaenoic acid production at low temperature. Biochem Biophys Res Commun. 1988 Jan 15;150(1):335–341. doi: 10.1016/0006-291x(88)90525-6. [DOI] [PubMed] [Google Scholar]
- Sumner J. L., Morgan E. D., Evans H. C. The effect of growth temperature on the fatty acid composition of fungi in the order Mucorales. Can J Microbiol. 1969 Jun;15(6):515–520. doi: 10.1139/m69-089. [DOI] [PubMed] [Google Scholar]



