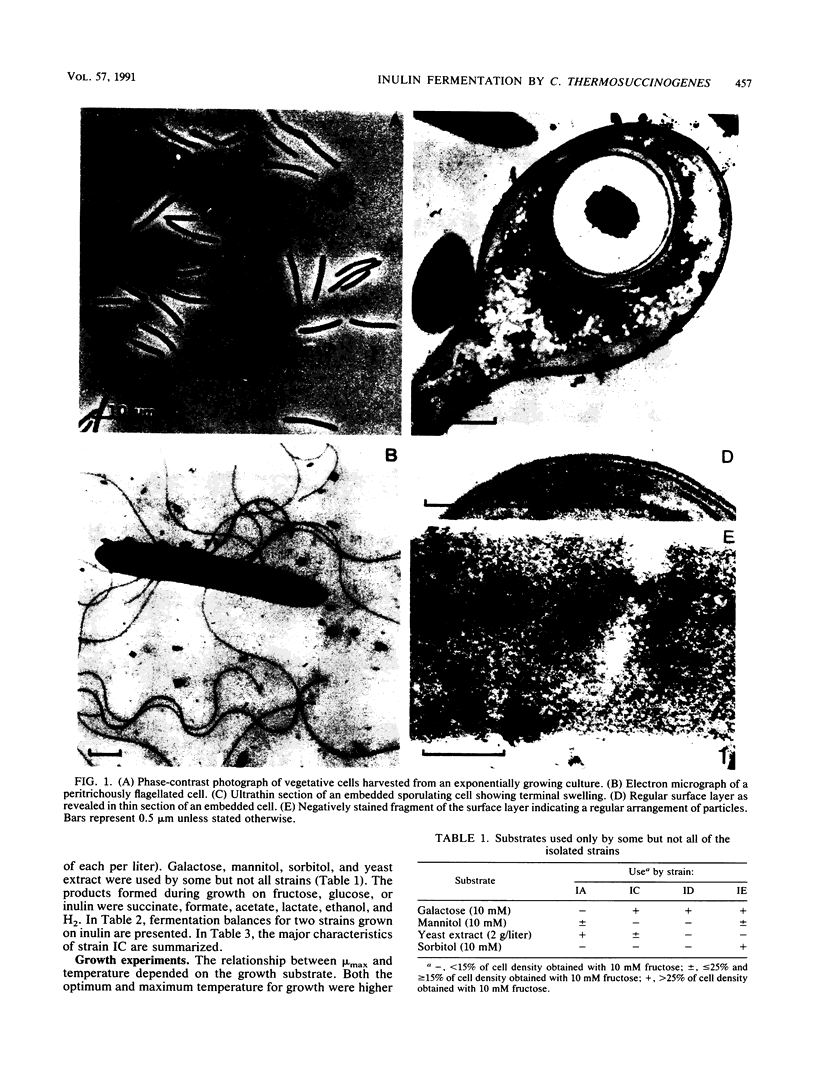
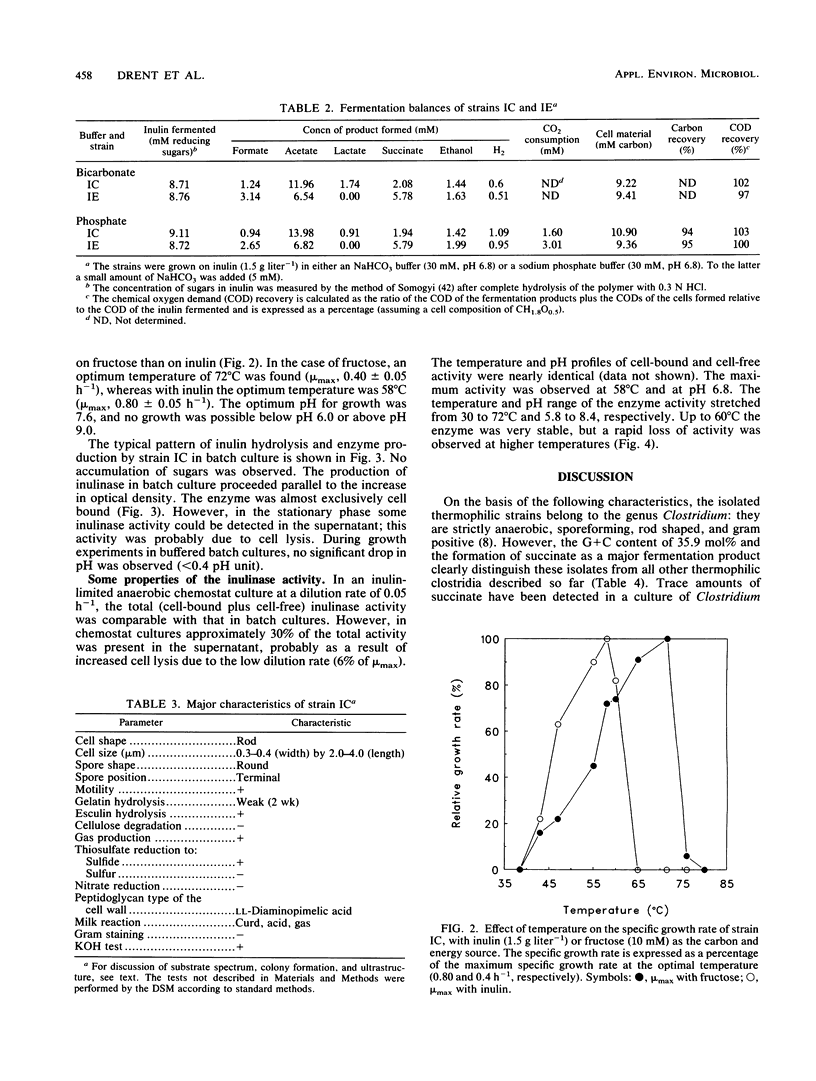
Abstract
Four closely related strains of thermophilic bacteria were isolated via enrichment in batch and continuous culture with inulin as the sole source of carbon and energy by using inoculations from various sources. These new strains were isolated from beet pulp from a sugar refinery, soil around a Jerusalem artichoke, fresh cow manure, and mud from a tropical pond in a botanical garden. The cells of this novel species of strictly anaerobic, gram-positive bacteria were rod shaped and nonmotile. Growth on inulin was possible between 40 and 65°C, with optimum growth at 58°C. All strains were capable of fermenting a large number of sugars. Formate, acetate, ethanol, lactate, H2, and succinate were the main organic fermentation products after growth on fructose, glucose, or inulin. Synthesis of inulinase in batch culture closely paralleled growth, and the enzyme was almost completely cell bound. Strain IC is described as the type strain of a new species, Clostridium thermosuccinogenes sp. nov., with a G+C content of 35.9 mol%.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allais J. J., Hoyos-Lopez G., Kammoun S., Baratti J. C. Isolation and characterization of thermophilic bacterial strains with inulinase activity. Appl Environ Microbiol. 1987 May;53(5):942–945. doi: 10.1128/aem.53.5.942-945.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Micro-organisms adapted to high temperatures. Nature. 1967 May 27;214(5091):882–885. doi: 10.1038/214882a0. [DOI] [PubMed] [Google Scholar]
- Freier Doris, Mothershed Cheryle P., Wiegel Juergen. Characterization of Clostridium thermocellum JW20. Appl Environ Microbiol. 1988 Jan;54(1):204–211. doi: 10.1128/aem.54.1.204-211.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A., de Bruijn J. M., Niedeveld C. J. Bacteria and yeasts as possible candidates for the production of inulinases and levanases. Antonie Van Leeuwenhoek. 1985;51(3):333–343. doi: 10.1007/BF02439942. [DOI] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Simultaneous and Enhanced Production of Thermostable Amylases and Ethanol from Starch by Cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1174–1181. doi: 10.1128/aem.49.5.1174-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo H., Cheng K. J., Costerton J. W. Interactions between Treponema bryantii and cellulolytic bacteria in the in vitro degradation of straw cellulose. Can J Microbiol. 1987 Mar;33(3):244–248. doi: 10.1139/m87-041. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Geerligs H. J., Sijtsma L., Veldkamp H. Competition for sulfate and ethanol among desulfobacter, desulfobulbus, and desulfovibrio species isolated from intertidal sediments. Appl Environ Microbiol. 1984 Feb;47(2):329–334. doi: 10.1128/aem.47.2.329-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y. Characterization of a Symbiotic Coculture of Clostridium thermohydrosulfuricum YM3 and Clostridium thermocellum YM4. Appl Environ Microbiol. 1990 Jan;56(1):37–42. doi: 10.1128/aem.56.1.37-42.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Fukui K., Kato K. Effect of bicarbonate on the growth of Actinobacillus actinomycetemcomitans in anaerobic fructose-limited chemostat culture. J Gen Microbiol. 1989 Dec;135(12):3485–3495. doi: 10.1099/00221287-135-12-3485. [DOI] [PubMed] [Google Scholar]
- Rouwenhorst R. J., Visser L. E., Van Der Baan A. A., Scheffers W. A., Van Dijken J. P. Production, Distribution, and Kinetic Properties of Inulinase in Continuous Cultures of Kluyveromyces marxianus CBS 6556. Appl Environ Microbiol. 1988 May;54(5):1131–1137. doi: 10.1128/aem.54.5.1131-1137.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Sandbeck K. A., Ward D. M. Temperature adaptations in the terminal processes of anaerobic decomposition of yellowstone national park and icelandic hot spring microbial mats. Appl Environ Microbiol. 1982 Oct;44(4):844–851. doi: 10.1128/aem.44.4.844-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme E. J., Derycke D. G. Microbial inulinases: fermentation process, properties, and applications. Adv Appl Microbiol. 1983;29:139–176. doi: 10.1016/s0065-2164(08)70356-3. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Ljungdahl L. G., Rawson J. R. Isolation from soil and properties of the extreme thermophile Clostridium thermohydrosulfuricum. J Bacteriol. 1979 Sep;139(3):800–810. doi: 10.1128/jb.139.3.800-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



