Abstract
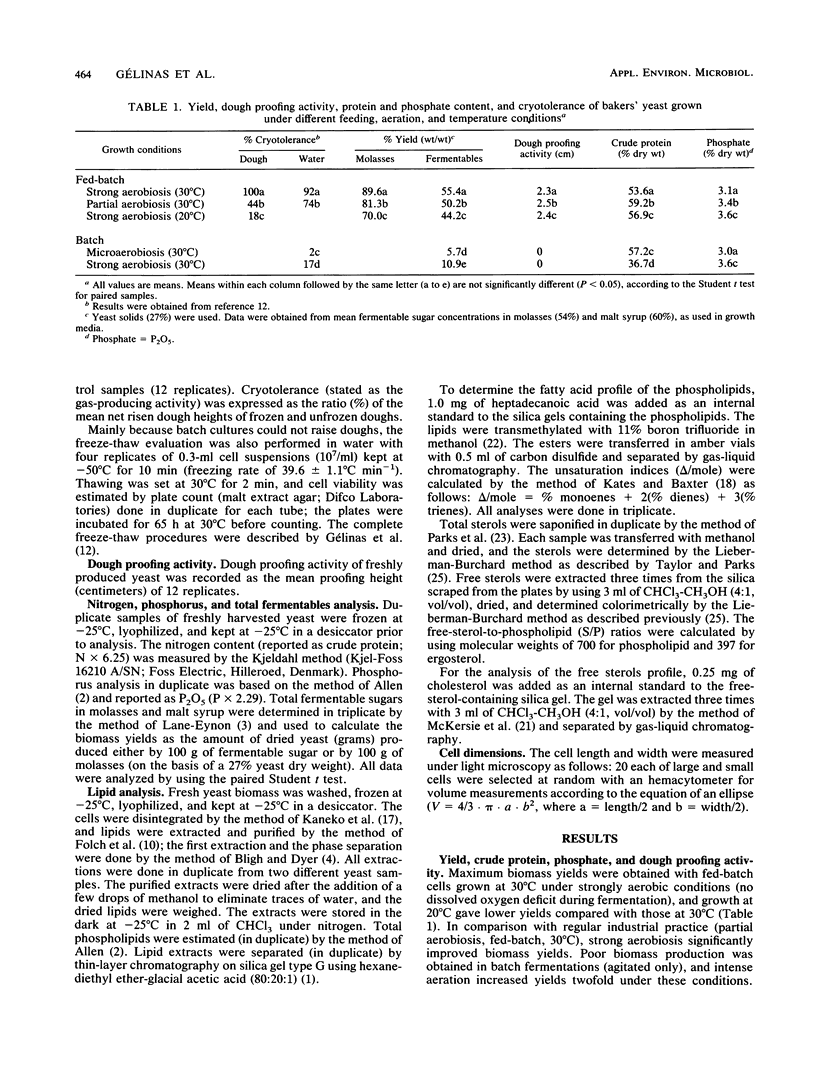
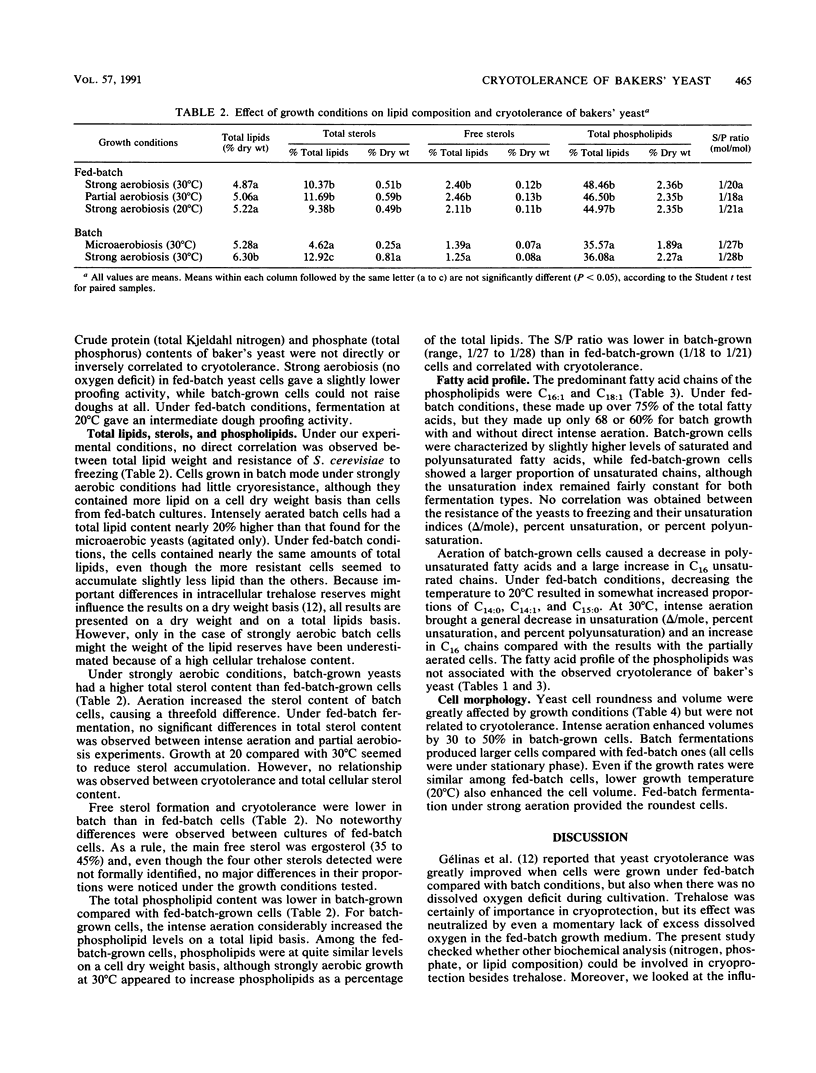
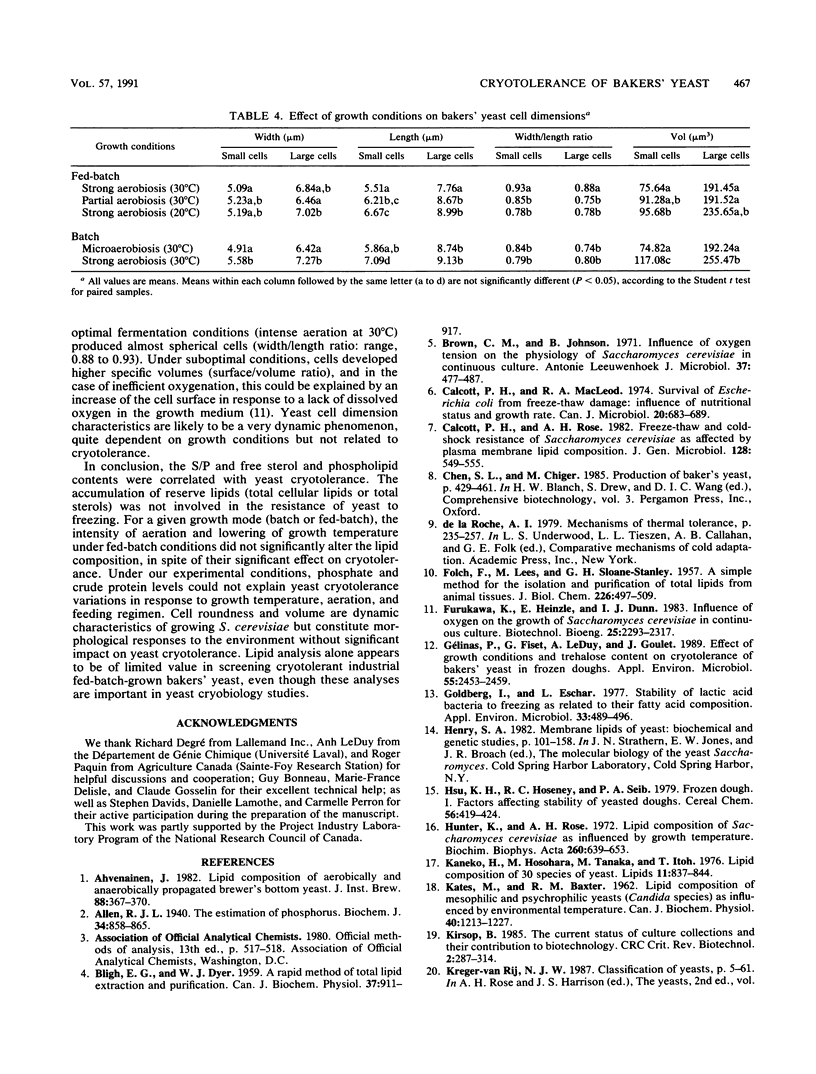
The relationship between lipid content and tolerance to freezing at −50°C was studied in Saccharomyces cerevisiae grown under batch or fed-batch mode and various aeration and temperature conditions. A higher free-sterol-to-phospholipid ratio as well as higher free sterol and phospholipid contents correlated with the superior cryoresistance in dough or in water of the fed-batch-grown compared with the batch-grown cells. For both growth modes, the presence of excess dissolved oxygen in the culture medium greatly improved yeast cryoresistance and trehalose content (P. Gélinas, G. Fiset, A. LeDuy, and J. Goulet, Appl. Environ. Microbiol. 26:2453-2459, 1989) without significantly changing the lipid profile. Under the batch or fed-batch modes, no correlation was found between the cryotolerance of bakers' yeast and the total cellular lipid content, the total sterol content, the phospholipid unsaturation index, the phosphate or crude protein content, or the yeast cell morphology (volume and roundness).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Johnson B. Influence of oxygen tension on the physiology of Saccharomyces cerevisiae in continuous culture. Antonie Van Leeuwenhoek. 1971;37(4):477–487. doi: 10.1007/BF02218518. [DOI] [PubMed] [Google Scholar]
- Calcott P. H., MacLeod R. A. Survival of Escherichia coli from freeze-thaw damage: influence of nutritional status and growth rate. Can J Microbiol. 1974 May;20(5):683–689. doi: 10.1139/m74-104. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldberg I., Eschar L. Stability of lactic Acid bacteria to freezing as related to their Fatty Acid composition. Appl Environ Microbiol. 1977 Mar;33(3):489–496. doi: 10.1128/aem.33.3.489-496.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas P., Fiset G., Leduy A., Goulet J. Effect of growth conditions and trehalose content on cryotolerance of bakers' yeast in frozen doughs. Appl Environ Microbiol. 1989 Oct;55(10):2453–2459. doi: 10.1128/aem.55.10.2453-2459.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K., Rose A. H. Lipid composition of Saccharomyces cerevisiae as influenced by growth temperature. Biochim Biophys Acta. 1972 Apr 18;260(4):639–653. doi: 10.1016/0005-2760(72)90013-6. [DOI] [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- Kaneko H., Hosohara M., Tanaka M., Itoh T. Lipid composition of 30 species of yeast. Lipids. 1976 Dec;11(12):837–844. doi: 10.1007/BF02532989. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Bottema C. D., Rodriguez R. J., Lewis T. A. Yeast sterols: yeast mutants as tools for the study of sterol metabolism. Methods Enzymol. 1985;111:333–346. doi: 10.1016/s0076-6879(85)11020-7. [DOI] [PubMed] [Google Scholar]
- Souzu H. Fluorescence polarization studies on Escherichia coli membrane stability and its relation to the resistance of the cell to freeze-thawing. I. Membrane stability in cells of differing growth phase. Biochim Biophys Acta. 1986 Oct 9;861(2):353–360. doi: 10.1016/0005-2736(86)90438-4. [DOI] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Metabolic interconversion of free sterols and steryl esters in Saccharomyces cerevisiae. J Bacteriol. 1978 Nov;136(2):531–537. doi: 10.1128/jb.136.2.531-537.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson C. B., Smith Q. J., Wheals A. E. Mean size of Saccharomyces cerevisiae cells growing at different growth rates [proceedings]. Biochem Soc Trans. 1978;6(3):564–565. doi: 10.1042/bst0060564. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Involvement of Plasma Membrane Alterations in Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1984 Jul;75(3):818–826. doi: 10.1104/pp.75.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vraná D. Daughter cells as an important factor in determining the physiological state of yeast populations. Biotechnol Bioeng. 1976 Mar;18(3):297–309. doi: 10.1002/bit.260180303. [DOI] [PubMed] [Google Scholar]
- Walker G. M., Duffus J. H. Magnesium ions and the control of the cell cycle in yeast. J Cell Sci. 1980 Apr;42:329–356. doi: 10.1242/jcs.42.1.329. [DOI] [PubMed] [Google Scholar]


