Abstract
We present a simple and rapid method for introducing exogenous DNA into a bacterium, Bacillus megaterium, utilizing the recently developed biolistic process. A suspension of B. megaterium was spread onto the surface of nonselective medium. Plasmid pUB110 DNA, which contains a gene that confers kanamycin resistance, was precipitated onto tungsten particles. Using a biolistic propulsion system, the coated particles were accelerated at high velocities into the B. megaterium recipient cells. Selection was done by use of an agar overlay containing 50 micrograms of kanamycin per ml. Antibiotic-resistant transformants were recovered from the medium interface after 72 h of incubation, and the recipient strain was shown to contain the delivered plasmid by agarose gel electrophoresis of isolated plasmid DNA. All strains of B. megaterium tested were successfully transformed by this method, although transformation efficiency varied among strains. Physical variables of the biolistic process and biological variables associated with the target cells were optimized, yielding greater than 10(4) transformants per treated plate. This is the first report of the biolistic transformation of a procaryote.
Full text
PDF

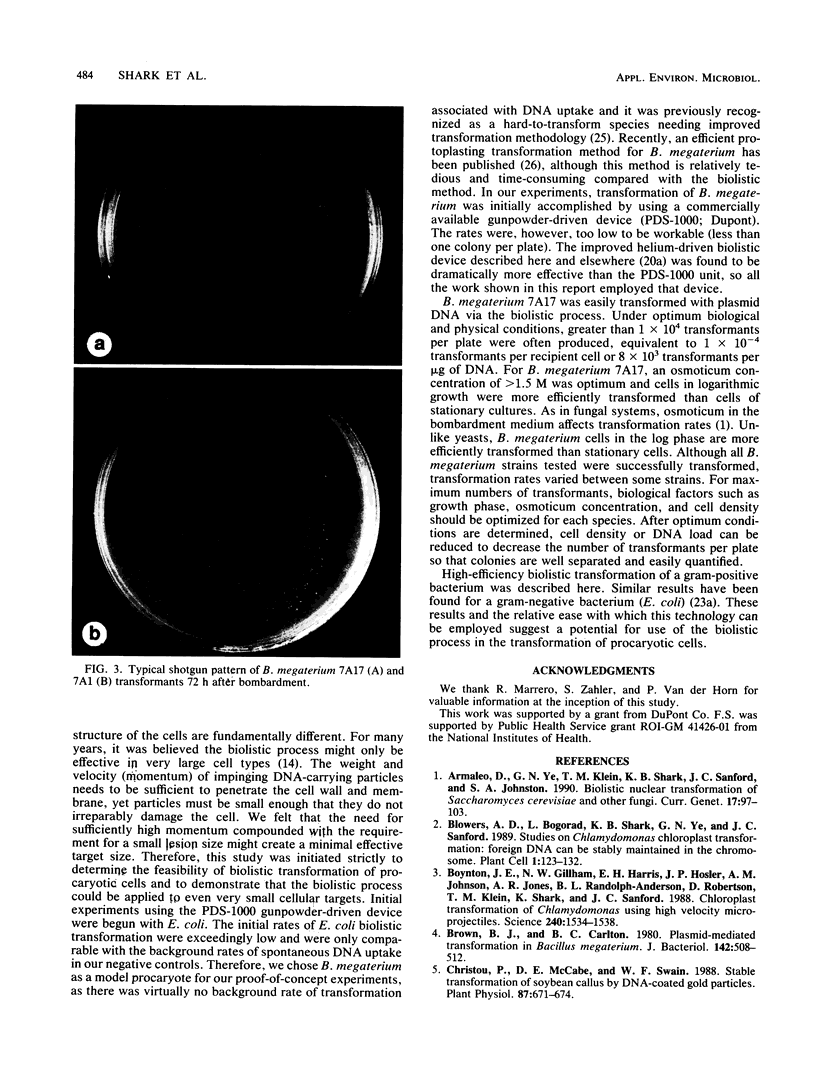

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armaleo D., Ye G. N., Klein T. M., Shark K. B., Sanford J. C., Johnston S. A. Biolistic nuclear transformation of Saccharomyces cerevisiae and other fungi. Curr Genet. 1990 Feb;17(2):97–103. doi: 10.1007/BF00312852. [DOI] [PubMed] [Google Scholar]
- Blowers A. D., Bogorad L., Shark K. B., Sanford J. C. Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell. 1989 Jan;1(1):123–132. doi: 10.1105/tpc.1.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Brown B. J., Carlton B. C. Plasmid-mediated transformation in Bacillus megaterium. J Bacteriol. 1980 May;142(2):508–512. doi: 10.1128/jb.142.2.508-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou P., McCabe D. E., Swain W. F. Stable Transformation of Soybean Callus by DNA-Coated Gold Particles. Plant Physiol. 1988 Jul;87(3):671–674. doi: 10.1104/pp.87.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H., Vivekananda J., Nielsen B. L., Ye G. N., Tewari K. K., Sanford J. C. Transient foreign gene expression in chloroplasts of cultured tobacco cells after biolistic delivery of chloroplast vectors. Proc Natl Acad Sci U S A. 1990 Jan;87(1):88–92. doi: 10.1073/pnas.87.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Sanford J. C., McMullin T. W. Plasmids can stably transform yeast mitochondria lacking endogenous mtDNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7288–7292. doi: 10.1073/pnas.85.19.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe J. C., Hess G. F., Franzen M. A., Vary P. S. Genetics of leucine biosynthesis in Bacillus megaterium QM B1551. J Bacteriol. 1984 Feb;157(2):454–459. doi: 10.1128/jb.157.2.454-459.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. M., Fromm M., Weissinger A., Tomes D., Schaaf S., Sletten M., Sanford J. C. Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4305–4309. doi: 10.1073/pnas.85.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchansky J. B., Muriana P. M., Klaenhammer T. R. Application of electroporation for transfer of plasmid DNA to Lactobacillus, Lactococcus, Leuconostoc, Listeria, Pediococcus, Bacillus, Staphylococcus, Enterococcus and Propionibacterium. Mol Microbiol. 1988 Sep;2(5):637–646. doi: 10.1111/j.1365-2958.1988.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Mallonee D. H., Speckman R. A. Transformation of Bacillus polymyxa with plasmid DNA. Appl Environ Microbiol. 1989 Oct;55(10):2517–2521. doi: 10.1128/aem.55.10.2517-2521.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. MECHANICAL GERMINATION OF BACTERIAL SPORES. Proc Natl Acad Sci U S A. 1960 Jan;46(1):118–128. doi: 10.1073/pnas.46.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A., Kalinowski J., Simon R., Seep-Feldhaus A. H., Pühler A. High-frequency conjugal plasmid transfer from gram-negative Escherichia coli to various gram-positive coryneform bacteria. J Bacteriol. 1990 Mar;172(3):1663–1666. doi: 10.1128/jb.172.3.1663-1666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Tersch M. A., Robbins H. L. Efficient cloning in Bacillus megaterium: comparison to Bacillus subtilis and Escherichia coli cloning hosts. FEMS Microbiol Lett. 1990 Aug;58(3):305–309. doi: 10.1111/j.1574-6968.1990.tb13994.x. [DOI] [PubMed] [Google Scholar]
- Zelenin A. V., Titomirov A. V., Kolesnikov V. A. Genetic transformation of mouse cultured cells with the help of high-velocity mechanical DNA injection. FEBS Lett. 1989 Feb 13;244(1):65–67. doi: 10.1016/0014-5793(89)81163-9. [DOI] [PubMed] [Google Scholar]