Abstract
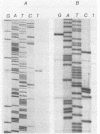
Two distinct regions of the Lactococcus lactis subsp. cremoris 9B4 plasmid p9B4-6, each of which specified bacteriocin production as well as immunity, have been sequenced and analyzed by deletion and frameshift mutation analyses. On a 1.8-kb ScaI-ClaI fragment specifying low antagonistic activity, three open reading frames (ORFs) were present, which were organized in an operon. The first two ORFs, containing 69 and 77 codons, respectively, were involved in bacteriocin activity, whereas the third ORF, containing 154 codons, was essential for immunity. Primer extension analysis indicated the presence of a promoter upstream of the ORFs. Two ORFs were present on a 1.3-kb ScaI-HindII fragment specifying high antagonistic activity. The first ORF, containing 75 codons, specified bacteriocin activity. The second ORF, containing 98 codons, specified immunity. The nucleotide sequences of both fragments upstream of the first ORFs as well as the first 20 bp of the first ORF of both bacteriocin operons appeared to be identical.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chopin A., Chopin M. C., Moillo-Batt A., Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984 May;11(3):260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Daeschel M. A., Klaenhammer T. R. Association of a 13.6-Megadalton Plasmid in Pediococcus pentosaceus with Bacteriocin Activity. Appl Environ Microbiol. 1985 Dec;50(6):1538–1541. doi: 10.1128/aem.50.6.1538-1541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey G. P. Plasmid associated with diplococcin production in Streptococcus. Appl Environ Microbiol. 1984 Oct;48(4):895–896. doi: 10.1128/aem.48.4.895-896.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Harmon K. S., McKay L. L. Restriction enzyme analysis of lactose and bacteriocin plasmids from Streptococcus lactis subsp. diacetylactis WM4 and cloning of BclI fragments coding for bacteriocin production. Appl Environ Microbiol. 1987 May;53(5):1171–1174. doi: 10.1128/aem.53.5.1171-1174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Neve H., Geis A., Teuber M. Conjugal transfer and characterization of bacteriocin plasmids in group N (lactic acid) streptococci. J Bacteriol. 1984 Mar;157(3):833–838. doi: 10.1128/jb.157.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Geis A., Kok J., Venema G. Cloning of two bacteriocin genes from a lactococcal bacteriocin plasmid. Appl Environ Microbiol. 1989 May;55(5):1187–1191. doi: 10.1128/aem.55.5.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelie D., Wösten H. A., Bron S., Oskam L., Venema G. Conjugal mobilization of streptococcal plasmid pMV158 between strains of Lactococcus lactis subsp. lactis. J Bacteriol. 1990 Jan;172(1):47–52. doi: 10.1128/jb.172.1.47-52.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelie D., van der Vossen J. M., Venema G. Effect of Plasmid Incompatibility on DNA Transfer to Streptococcus cremoris. Appl Environ Microbiol. 1988 Apr;54(4):865–871. doi: 10.1128/aem.54.4.865-871.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen J. M., van der Lelie D., Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987 Oct;53(10):2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]