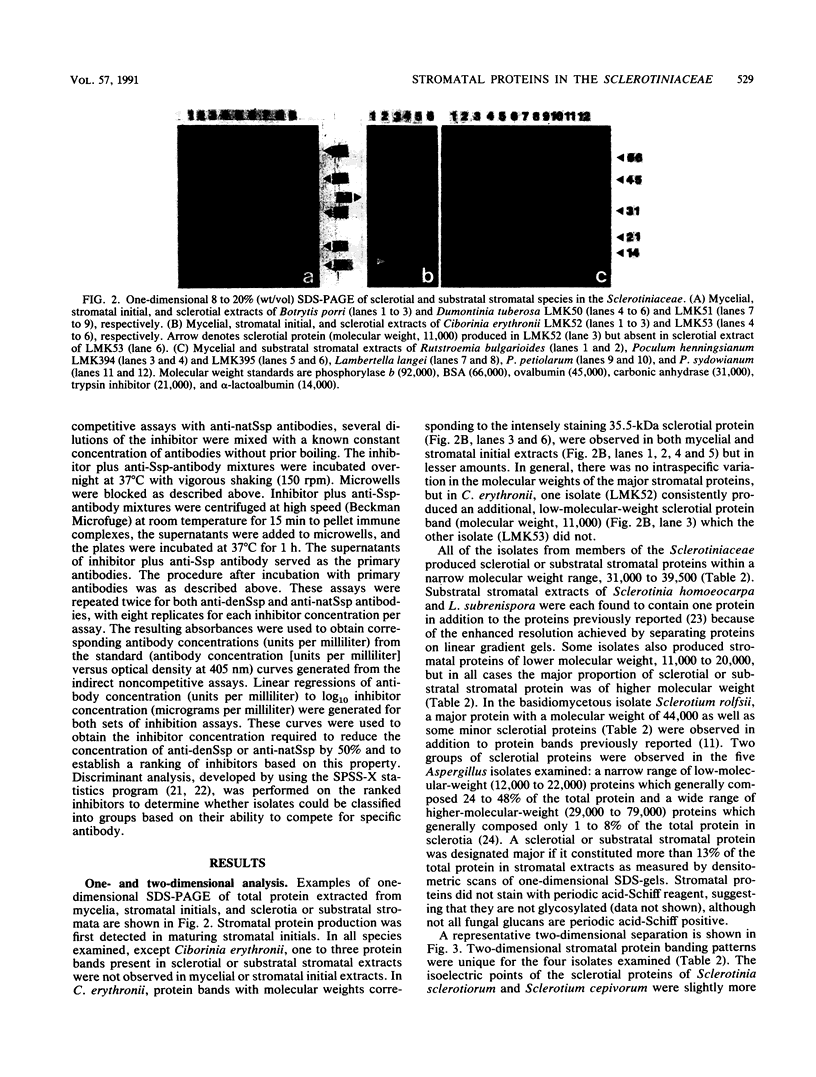
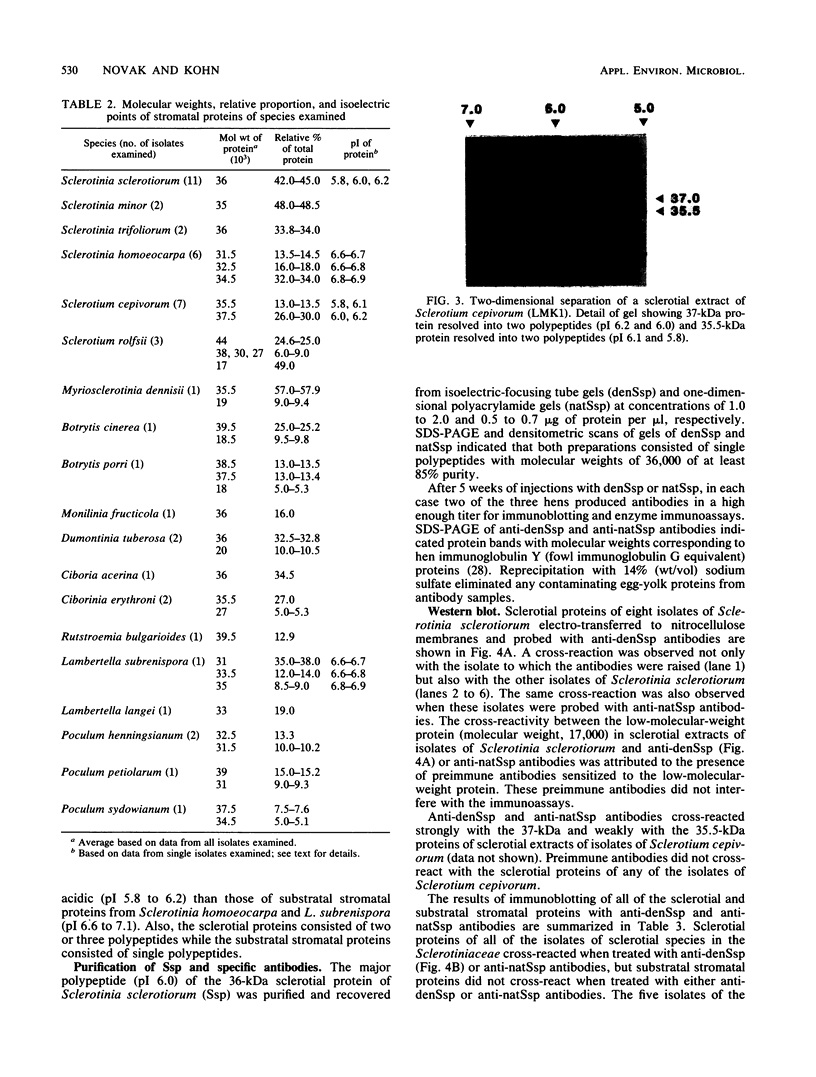
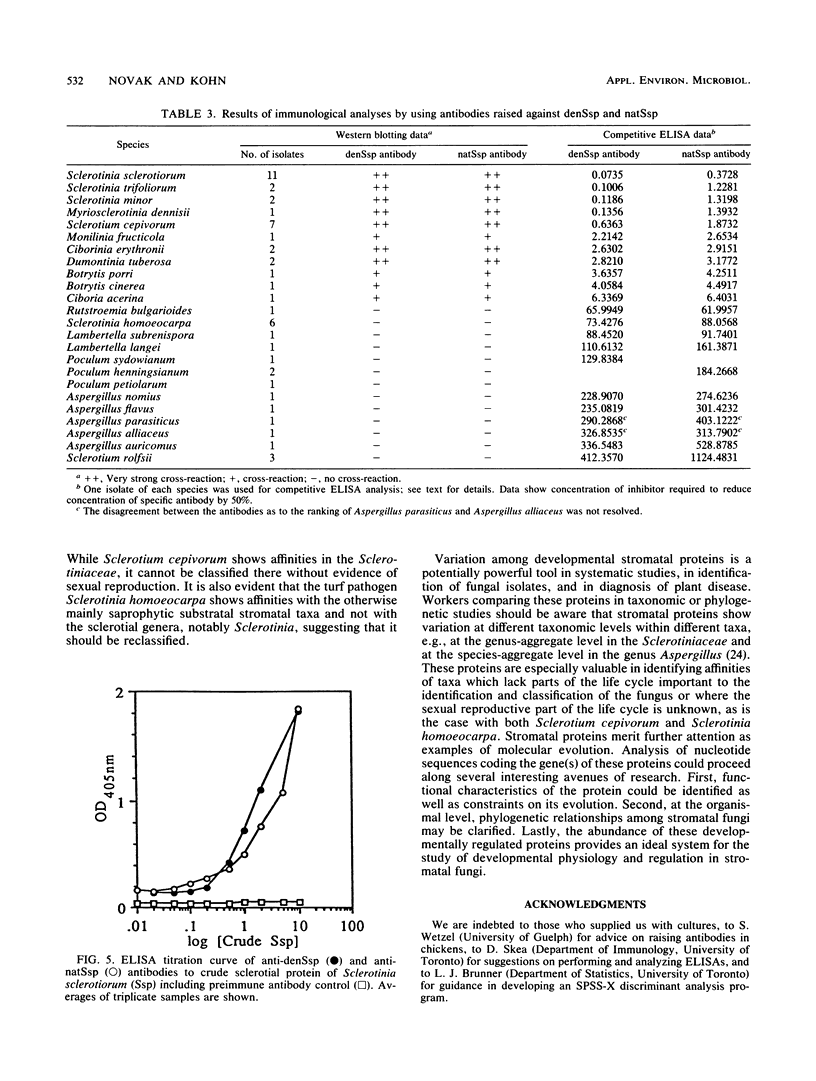
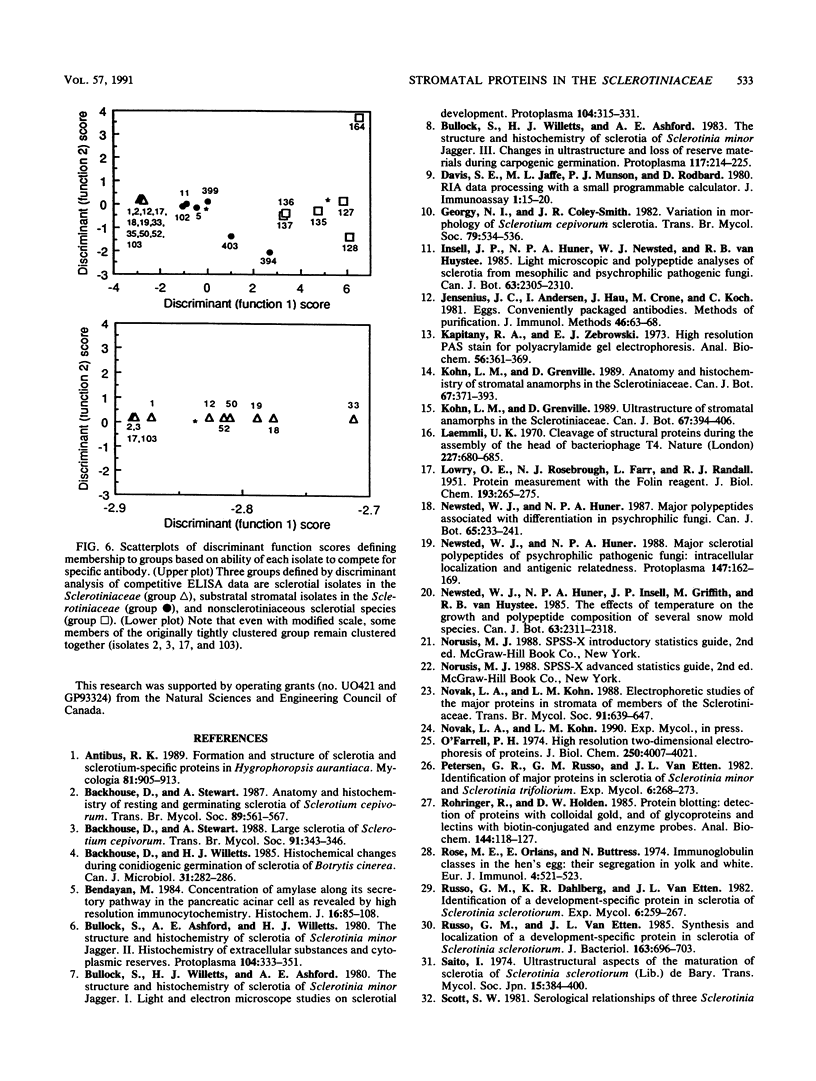
Abstract
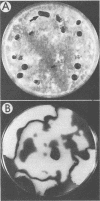
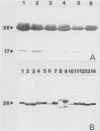
The fungal stroma is a distinct developmental stage, a compact mass of hyphal cells enveloped by a melanized layer of rind cells which is produced from vegetative mycelium. Two types of stromata that are characteristic of members of the Sclerotiniaceae but are also produced in a wide range of other fungi, i.e., the determinate tuberlike sclerotium and the indeterminate platelike substratal stroma, were compared in these studies. Developmental proteins found in determinate sclerotial and indeterminate substratal stromata, but not in mycelia, were characterized and compared in 52 isolates of fungi, both ascomycetes (including 18 species in the Sclerotiniaceae and 5 species of Aspergillus) and the basidiomycete Sclerotium rolfsii. One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis of mycelial, stromatal initial, and stromatal extracts demonstrated that all members of the Sclerotiniaceae produced proteins unique to stromatal extracts within a molecular weight range of 31,000 to 39,500 which composed 13 to 58% of the total protein in stromata. Proteins unique to the sclerotial stage were also produced in Sclerotium rolfsii and the Aspergillus species but within a generally lower-molecular-weight range of 11,000 to 30,000. The proteins were then characterized by two-dimensional electrophoresis to determine the number and isoelectric point of polypeptides composing each protein. Polyclonal antibodies were raised to the major 36-kDa sclerotial protein of Sclerotinia sclerotiorum (Ssp). Immunoblots demonstrated that all sclerotial proteins from species in the Sclerotiniaceae cross-reacted with anti-Ssp antibodies, while no cross-reaction was observed with proteins from substratal stromatal species in the Sclerotiniaceae, sclerotial species of Aspergillus, or Sclerotium rolfsii. Results of discriminant analysis of the data from competitive inhibition enzyme-linked immunosorbent assays were consistent with the results of immunoblotting. Three groupings, sclerotial species in the Sclerotiniaceae, substratal stromatal species in the family, and sclerotial species outside the family, were delimited on the basis of relative decreasing ability to compete for anti-Ssp antibody. These data demonstrate that stromatal proteins differ among different taxonomic groups of fungi and suggest that the Sclerotiniaceae may include two distinct lineages of genera.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendayan M. Concentration of amylase along its secretory pathway in the pancreatic acinar cell as revealed by high resolution immunocytochemistry. Histochem J. 1984 Jan;16(1):85–108. doi: 10.1007/BF01003438. [DOI] [PubMed] [Google Scholar]
- Davis S. E., Munson P. J., Jaffe M. L., Rodbard D. Radioimmunoassay data processing with a small programmable calculator. J Immunoassay. 1980;1(1):15–25. doi: 10.1080/01971528008055773. [DOI] [PubMed] [Google Scholar]
- Jensenius J. C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46(1):63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rohringer R., Holden D. W. Protein blotting: detection of proteins with colloidal gold, and of glycoproteins and lectins with biotin-conjugated and enzyme probes. Anal Biochem. 1985 Jan;144(1):118–127. doi: 10.1016/0003-2697(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Orlans E., Buttress N. Immunoglobulin classes in the hen's egg: their segregation in yolk and white. Eur J Immunol. 1974 Jul;4(7):521–523. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- Russo G. M., Van Etten J. L. Synthesis and localization of a development-specific protein in sclerotia of Sclerotinia sclerotiorum. J Bacteriol. 1985 Aug;163(2):696–703. doi: 10.1128/jb.163.2.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]