Abstract
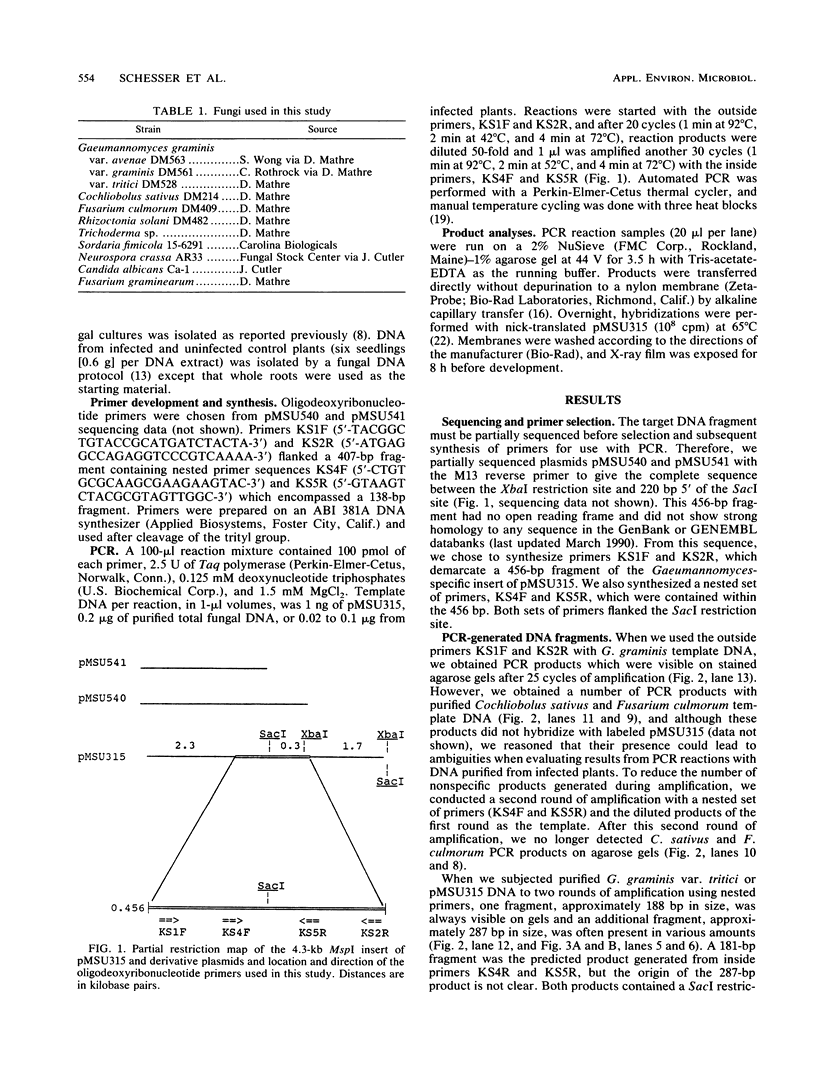
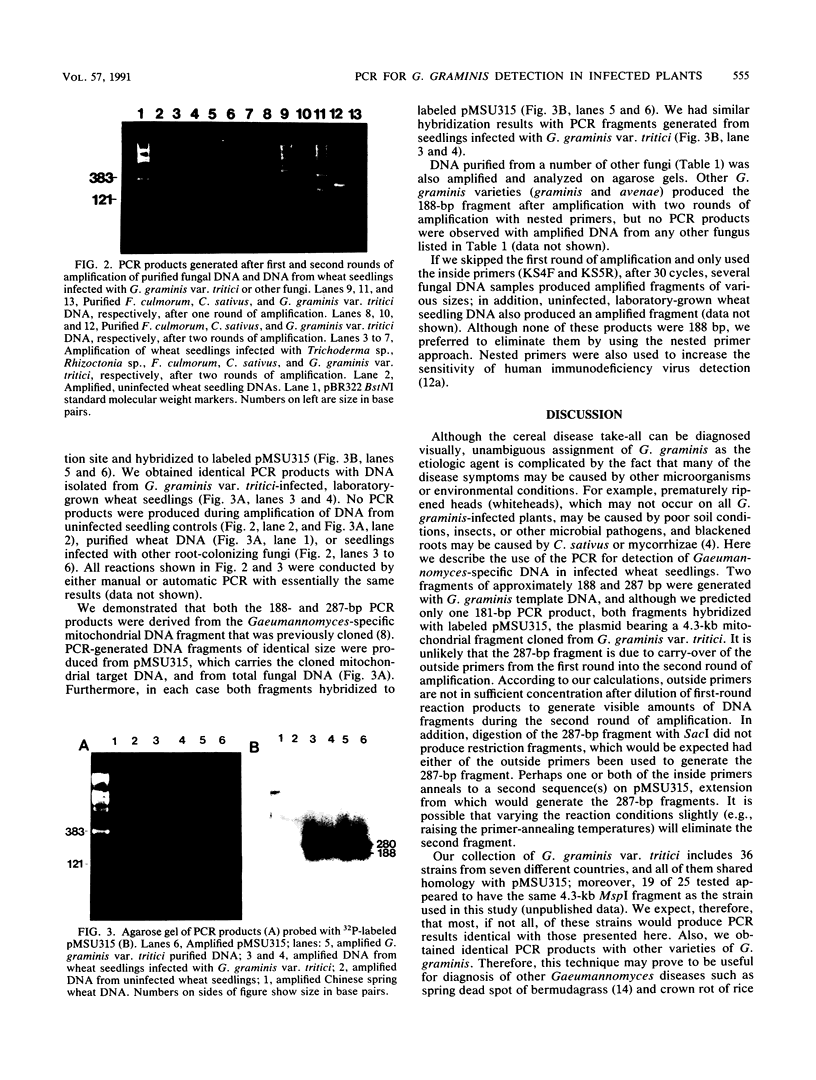
Gaeumannomyces graminis, the causative agent of take-all disease of wheat, barley, and oats, was detected in infected wheat seedlings by using the polymerase chain reaction to amplify Gaeumannomyces-specific DNA fragments. Nested primers and two rounds of amplification were used to amplify two fragments, approximately 287 and 188 bp in size, from G. graminis-infected wheat seedlings. The use of nested primers greatly decreased the number of nonspecific amplification products. Polymerase chain reaction products were not obtained with DNA from seedlings infected with several other phytopathogenic fungi or with DNA from uninfected seedlings. Amplified products were visualized on agarose gels, and their identities were confirmed by DNA hybridization. This method did not require culturing the fungus and has potential for detecting G. graminis in infested wheat, barley, or oat fields.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. M. DNA Probe for Identification of the Take-All Fungus, Gaeumannomyces graminis. Appl Environ Microbiol. 1989 Feb;55(2):284–288. doi: 10.1128/aem.55.2.284-288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Smith D. B., Foote S. J., Samaras N., Peterson M. G. Colorimetric detection of specific DNA segments amplified by polymerase chain reactions. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2423–2427. doi: 10.1073/pnas.86.7.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo F., Salvi R., Torchia P. Highly sensitive and fast detection of Phoma tracheiphila by polymerase chain reaction. Appl Microbiol Biotechnol. 1990 Feb;32(5):572–576. doi: 10.1007/BF00173730. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]