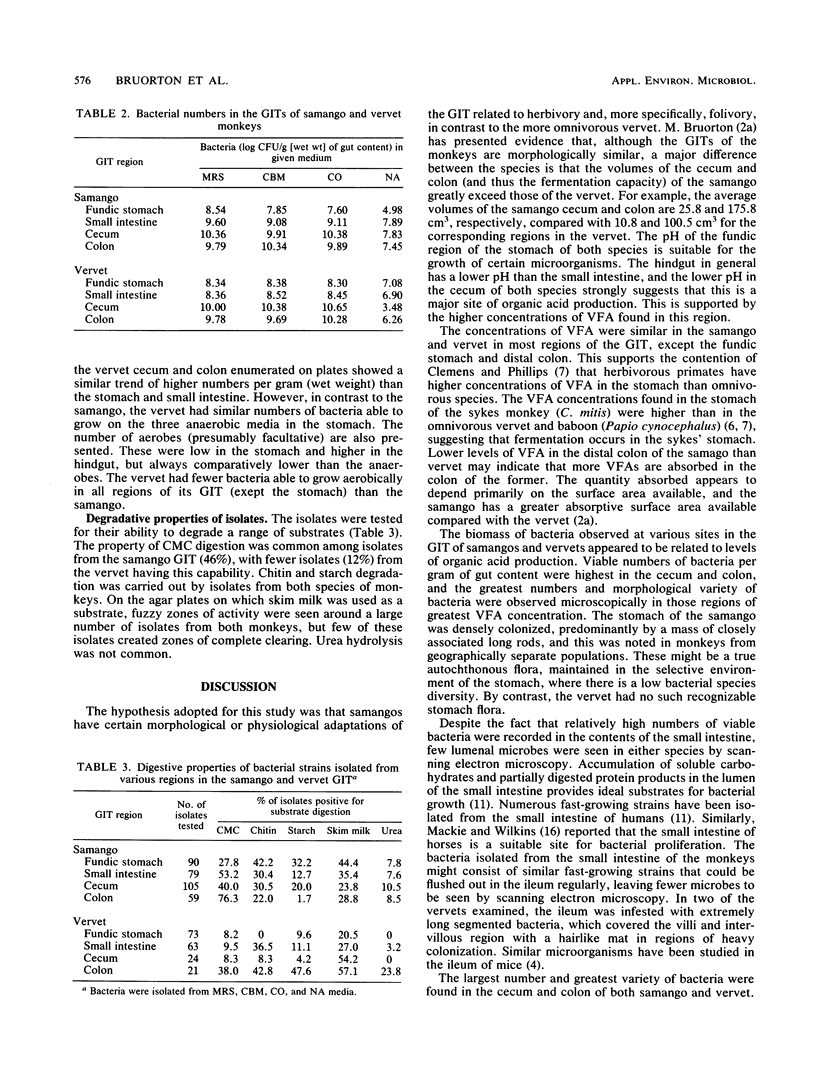
Abstract
The microflora in the gastrointestinal tracts of wild vervet and samango monkeys (Cercopithecus aethiops and C. mitis, respectively) were studied, using fermentation acid analysis, electron microscopy, and culturing methods. The diets of the two species of monkey differ considerably, with that of the samango including a greater proportion of cellulose-rich leaf material, and this is reflected in the microflora. Volatile fatty acid measurements along the gut of both species showed that these end products of bacterial metabolism were concentrated in the cecum and colon. Electron microscopy indicated that morphologically similar bacteria were present in the cecum and colon of both species, but the samango possessed a distinct stomach microflora. Bacteria in the lumina of the four main regions of the gut of the monkeys (stomach, small intestine, cecum, and colon) were plated on a number of anaerobic media (Mann, Rogosa, and Sharp; clostridial basal; and complex media). The cecum and colon were found to contain higher numbers of microbes per gram (wet weight) of gut content than the stomach and small intestine. Microbial isolates were able to catabolize carboxymethyl cellulose and other polymers. This may aid the monkeys, particularly samangos, in the digestion of fibrous dietary components such as leaves.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauchop T., Martucci R. W. Ruminant-like digestion of the langur monkey. Science. 1968 Aug 16;161(3842):698–700. doi: 10.1126/science.161.3842.698. [DOI] [PubMed] [Google Scholar]
- Bauchop T. Stomach microbiology of primates. Annu Rev Microbiol. 1971;25:429–436. doi: 10.1146/annurev.mi.25.100171.002241. [DOI] [PubMed] [Google Scholar]
- Chase D. G., Erlandsen S. L. Evidence for a complex life cycle and endospore formation in the attached, filamentous, segmented bacterium from murine ileum. J Bacteriol. 1976 Jul;127(1):572–583. doi: 10.1128/jb.127.1.572-583.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers D. J., Hladik C. M. Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J Morphol. 1980 Dec;166(3):337–386. doi: 10.1002/jmor.1051660306. [DOI] [PubMed] [Google Scholar]
- Cowley H. M., Hill R. R. "Intestinal spirochaetosis" of the vervet monkey. Onderstepoort J Vet Res. 1985 Mar;52(1):47–50. [PubMed] [Google Scholar]
- Dunbar R. I., Dunbar E. P. Ecological relations and niche separation between sympatric terrestrial primates in Ethiopia. Folia Primatol (Basel) 1974;21(1):36–60. doi: 10.1159/000155595. [DOI] [PubMed] [Google Scholar]
- Kavanagh M. The diet and feeding behaviour of Cercopithecus aethiops tantalus. Folia Primatol (Basel) 1978;30(1):30–63. doi: 10.1159/000155854. [DOI] [PubMed] [Google Scholar]
- Leach W. D., Lee A., Stubbs R. P. Localization of bacteria in the gastrointestinal tract: a possible explanation of intestinal spirochaetosis. Infect Immun. 1973 Jun;7(6):961–972. doi: 10.1128/iai.7.6.961-972.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Phillips M. Isolation and cultivation of spirochetes and other spiral-shaped bacteria associated with the cecal mucosa of rats and mice. Appl Environ Microbiol. 1978 Mar;35(3):610–613. doi: 10.1128/aem.35.3.610-613.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Wilkins C. A. Enumeration of anaerobic bacterial microflora of the equine gastrointestinal tract. Appl Environ Microbiol. 1988 Sep;54(9):2155–2160. doi: 10.1128/aem.54.9.2155-2160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. J., Nickel J. C., Cheng K. J., Costerton J. W. The ecology and pathogenicity of urease-producing bacteria in the urinary tract. Crit Rev Microbiol. 1988;16(1):37–79. doi: 10.3109/10408418809104467. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Jervis H. R., Nakazawa H., Robinson D. M. Spiral-shaped organisms on the surface colonic epithelium of the monkey and man. Am J Clin Nutr. 1974 Nov;27(11):1287–1296. doi: 10.1093/ajcn/27.11.1287. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Zeller J. A. Scanning electron microscopic observations on the surface of the normal and spirochete-infested colonic mucosa of the rhesus monkey. J Ultrastruct Res. 1972 Aug;40(3):313–324. doi: 10.1016/s0022-5320(72)90103-7. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



