Abstract
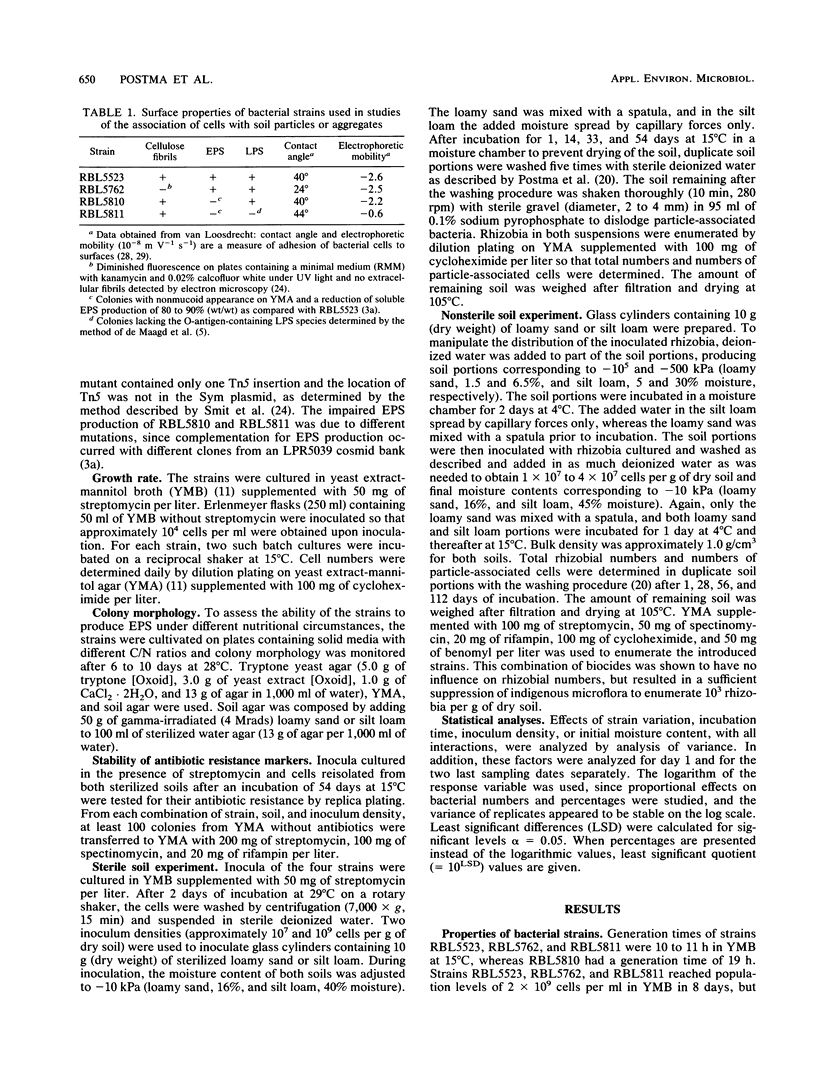
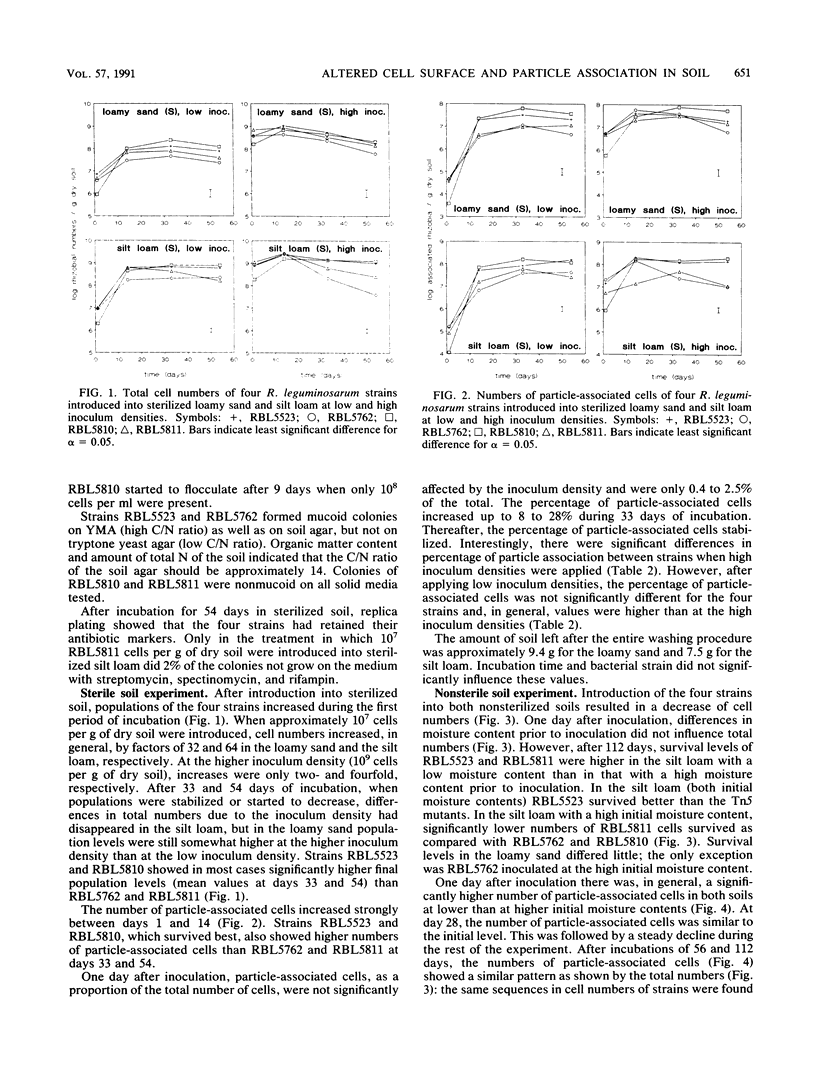
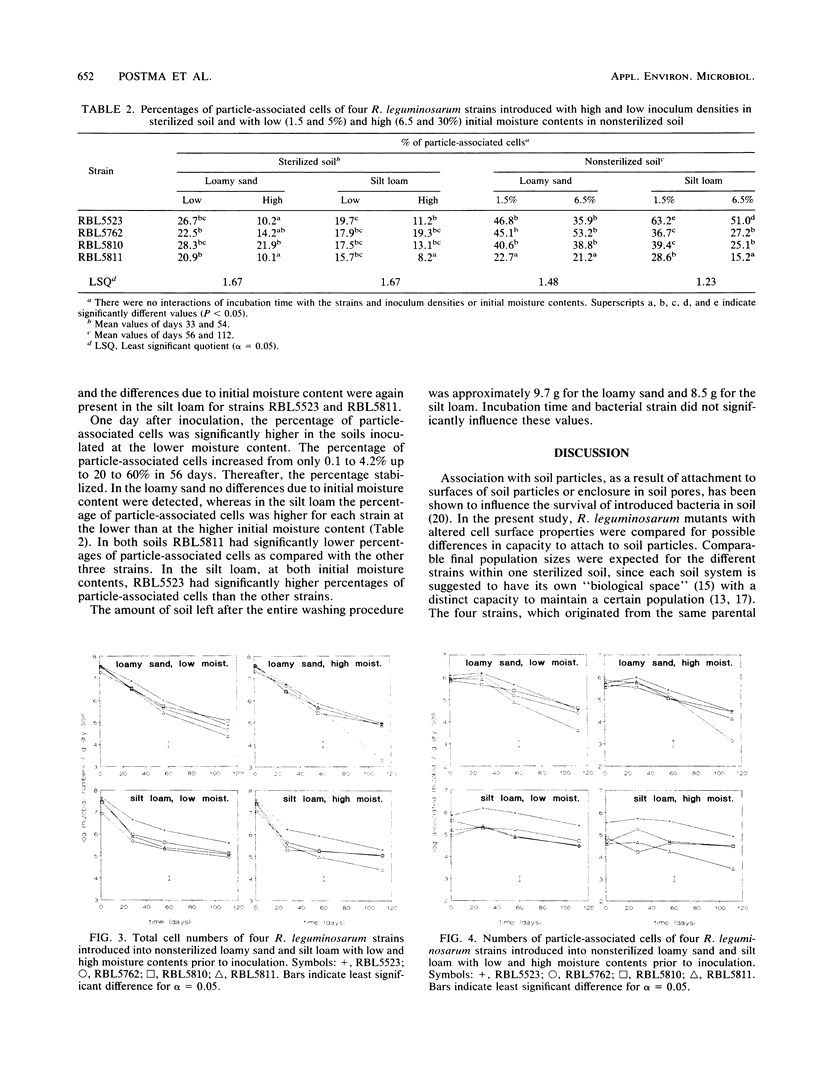
The influence of cell surface properties on attachment to soil particles and on population dynamics of introduced bacteria was studied in sterilized and nonsterilized loamy sand and silt loam. Rhizobium leguminosarum RBL5523 and three Tn5 mutants (RBL5762, RBL5810, and RBL5811) with altered cell surface properties were used. Cellulose fibrils were not produced by RBL5762. Both RBL5810 and RBL5811 produced 80 to 90% less soluble exopolysaccharides and RBL5811 had, in addition, an altered lipopolysaccharide composition. In sterilized soil the total number of cells as well as the number of particle-associated cells of RBL5523 and RBL5810 were, in general, higher as compared with cell numbers of RBL5762 and RBL5811. Differences between strains in percentage of particle-associated cells in sterilized soil were only found at high inoculum densities, when populations increased little. In the nonsterilized silt loam, final population sizes, as well as numbers of particle-associated cells, of the parental strain (RBL5523) were higher than those of strains with altered cell surface properties after 56 and 112 days of incubation. But in general, differences in survival among the strains were not very marked. The importance of association with soil particles or aggregates for the survival of introduced cells was affirmed by the pronounced increase of the percentage of particle-associated cells during incubation in nonsterilized as well as sterilized soil. However, no clear relation among altered cell surface properties, particle association, and survival was found.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill D. L., Casida L. E. Attachment to autoclaved soil of bacterial cells from pure cultures of soil isolates. Appl Environ Microbiol. 1979 May;37(5):1031–1037. doi: 10.1128/aem.37.5.1031-1037.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deflaun M. F., Tanzer A. S., McAteer A. L., Marshall B., Levy S. B. Development of an Adhesion Assay and Characterization of an Adhesion-Deficient Mutant of Pseudomonas fluorescens. Appl Environ Microbiol. 1990 Jan;56(1):112–119. doi: 10.1128/aem.56.1.112-119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol A. E., Hollingsworth R. I., Dazzo F. B. Alteration of surface properties in a Tn5 mutant strain of Rhizobium trifolii 0403. J Bacteriol. 1987 Mar;169(3):1161–1167. doi: 10.1128/jb.169.3.1161-1167.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeda D. P., Liu K. C., Casida L. E., Jr Colonization of soil by Arthrobacter and Pseudomonas under varying conditions of water and nutrient availability as studied by plate counts and transmission electron microscopy. Appl Environ Microbiol. 1976 Apr;31(4):551–561. doi: 10.1128/aem.31.4.551-561.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Yamaguchi M. Fractionation and Estimation of Particle-Attached and Unattached Bradyrhizobium japonicum Strains in Soils. Appl Environ Microbiol. 1986 Oct;52(4):911–914. doi: 10.1128/aem.52.4.911-914.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma J., Hok-A-Hin C. H., van Veen J. A. Role of Microniches in Protecting Introduced Rhizobium leguminosarum biovar trifolii against Competition and Predation in Soil. Appl Environ Microbiol. 1990 Feb;56(2):495–502. doi: 10.1128/aem.56.2.495-502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1987 Sep;169(9):4294–4301. doi: 10.1128/jb.169.9.4294-4301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Bacterial exopolysaccharides. Adv Microb Physiol. 1972;8:143–213. doi: 10.1016/s0065-2911(08)60190-3. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



