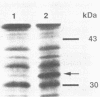
Abstract
A lambda recombinant phage expressing beta-mannanase activity in Escherichia coli has been isolated from a genomic library of the extremely thermophilic anaerobe "Caldocellum saccharolyticum." The gene was cloned into pBR322 on a 5-kb BamHI fragment, and its location was obtained by deletion analysis. The sequence of a 2.1-kb fragment containing the mannanase gene has been determined. One open reading frame was found which could code for a protein of Mr 38,904. The mannanase gene (manA) was overexpressed in E. coli by cloning the gene downstream from the lacZ promoter of pUC18. The enzyme was most active at pH 6 and 80 degrees C and degraded locust bean gum, guar gum, Pinus radiata glucomannan, and konjak glucomannan. The noncoding region downstream from the mannanase gene showed strong homology to celB, a gene coding for a cellulase from the same organism, suggesting that the manA gene might have been inserted into its present position on the "C. saccharolyticum" genome by homologous recombination.
Full text
PDF
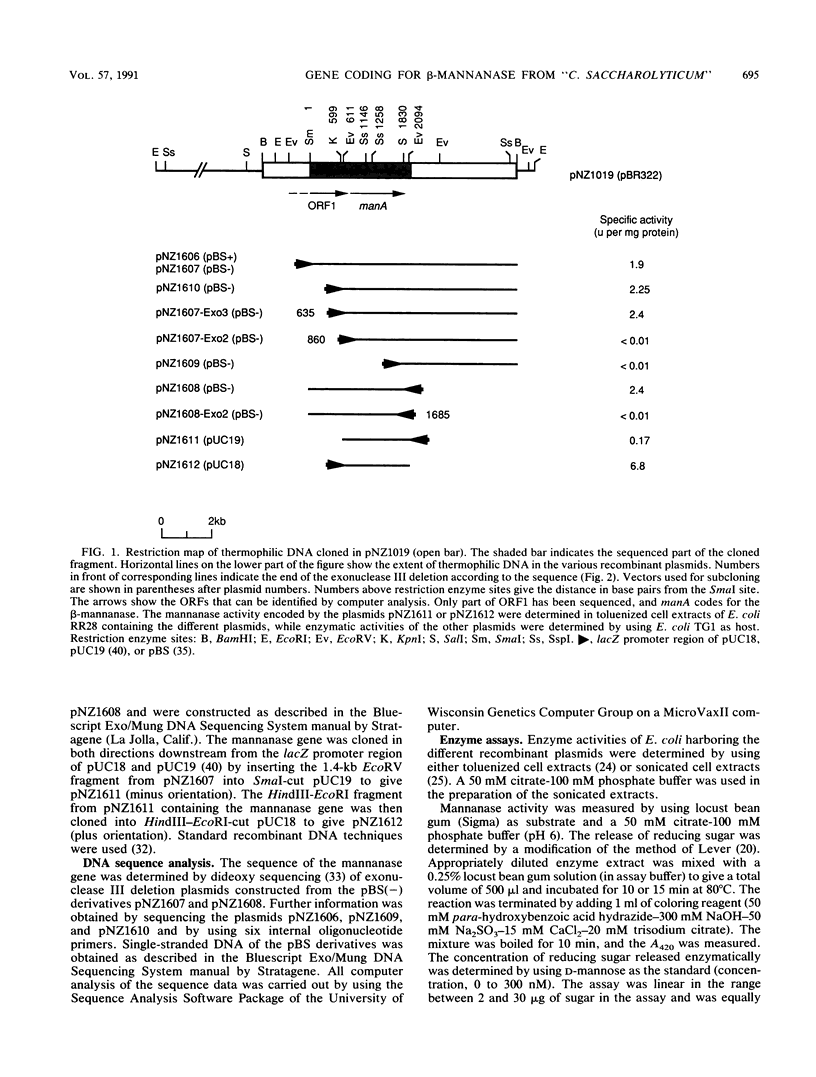





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akino T., Kato C., Horikoshi K. Two Bacillus beta-mannanases having different COOH termini are produced in Escherichia coli carrying pMAH5. Appl Environ Microbiol. 1989 Dec;55(12):3178–3183. doi: 10.1128/aem.55.12.3178-3183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A., Ward O. P. Hemicellulases of Bacillus species: preliminary comparative studies on production and properties of mannanases and galactanases. J Appl Bacteriol. 1990 Mar;68(3):253–261. doi: 10.1111/j.1365-2672.1990.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Bergquist P. L., Love D. R., Croft J. E., Streiff M. B., Daniel R. M., Morgan W. H. Genetics and potential biotechnological applications of thermophilic and extremely thermophilic micro-organisms. Biotechnol Genet Eng Rev. 1987;5:199–244. doi: 10.1080/02648725.1987.10647838. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Croft J. E., Love D. R., Bergquist P. L. Expression of leucine genes from an extremely thermophilic bacterium in Escherichia coli. Mol Gen Genet. 1987 Dec;210(3):490–497. doi: 10.1007/BF00327202. [DOI] [PubMed] [Google Scholar]
- Gilbert H. J., Sullivan D. A., Jenkins G., Kellett L. E., Minton N. P., Hall J. Molecular cloning of multiple xylanase genes from Pseudomonas fluorescens subsp. cellulosa. J Gen Microbiol. 1988 Dec;134(12):3239–3247. doi: 10.1099/00221287-134-12-3239. [DOI] [PubMed] [Google Scholar]
- Grépinet O., Chebrou M. C., Béguin P. Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum. J Bacteriol. 1988 Oct;170(10):4582–4588. doi: 10.1128/jb.170.10.4582-4588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Günther I., Binder F. A novel cloning vector for the direct selection of recombinant DNA in E. coli. Gene. 1982 Sep;19(2):231–234. doi: 10.1016/0378-1119(82)90011-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langsford M. L., Gilkes N. R., Singh B., Moser B., Miller R. C., Jr, Warren R. A., Kilburn D. G. Glycosylation of bacterial cellulases prevents proteolytic cleavage between functional domains. FEBS Lett. 1987 Dec 10;225(1-2):163–167. doi: 10.1016/0014-5793(87)81150-x. [DOI] [PubMed] [Google Scholar]
- Lever M. Colorimetric and fluorometric carbohydrate determination with p-hydroxybenzoic acid hydrazide. Biochem Med. 1973 Apr;7(2):274–281. doi: 10.1016/0006-2944(73)90083-5. [DOI] [PubMed] [Google Scholar]
- Love D. R., Fisher R., Bergquist P. L. Sequence structure and expression of a cloned beta-glucosidase gene from an extreme thermophile. Mol Gen Genet. 1988 Jul;213(1):84–92. doi: 10.1007/BF00333402. [DOI] [PubMed] [Google Scholar]
- Lüthi E., Jasmat N. B., Bergquist P. L. Overproduction of an acetylxylan esterase from the extreme thermophile "Caldocellum saccharolyticum" in Escherichia coli. Appl Microbiol Biotechnol. 1990 Nov;34(2):214–219. doi: 10.1007/BF00166783. [DOI] [PubMed] [Google Scholar]
- Lüthi E., Jasmat N. B., Bergquist P. L. Xylanase from the extremely thermophilic bacterium "Caldocellum saccharolyticum": overexpression of the gene in Escherichia coli and characterization of the gene product. Appl Environ Microbiol. 1990 Sep;56(9):2677–2683. doi: 10.1128/aem.56.9.2677-2683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi E., Love D. R., McAnulty J., Wallace C., Caughey P. A., Saul D., Bergquist P. L. Cloning, sequence analysis, and expression of genes encoding xylan-degrading enzymes from the thermophile "Caldocellum saccharolyticum". Appl Environ Microbiol. 1990 Apr;56(4):1017–1024. doi: 10.1128/aem.56.4.1017-1024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul D. J., Williams L. C., Grayling R. A., Chamley L. W., Love D. R., Bergquist P. L. celB, a gene coding for a bifunctional cellulase from the extreme thermophile "Caldocellum saccharolyticum". Appl Environ Microbiol. 1990 Oct;56(10):3117–3124. doi: 10.1128/aem.56.10.3117-3124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul D. J., Williams L. C., Love D. R., Chamley L. W., Bergquist P. L. Nucleotide sequence of a gene from Caldocellum saccharolyticum encoding for exocellulase and endocellulase activity. Nucleic Acids Res. 1989 Jan 11;17(1):439–439. doi: 10.1093/nar/17.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Wauven C., Piérard A., Kley-Raymann M., Haas D. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J Bacteriol. 1984 Dec;160(3):928–934. doi: 10.1128/jb.160.3.928-934.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. A., Elzanowski A., Yeh L. S., Barker W. C. Homologues of catalytic domains of Cellulomonas glucanases found in fungal and Bacillus glycosidases. FEMS Microbiol Lett. 1989 May;50(1-2):167–172. doi: 10.1016/0378-1097(89)90479-5. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]