Abstract
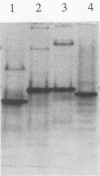
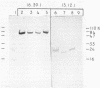
Crude extracts from 14 species of sulfate-reducing bacteria comprising the genera Desulfovibrio, Desulfotomaculum, Desulfobulbus, and Desulfosarcina and from three species of sulfide-oxidizing bacteria were tested in an enzyme-linked immunosorbent assay with polyclonal antisera to adenosine 5′-phosphosulfate reductase from Desulfovibrio desulfuricans G100A. The results showed that extracts from Desulfovibrio species were all highly cross-reactive, whereas extracts from the other sulfate-reducing genera showed significantly less cross-reaction. An exception was Desulfotomaculum orientis, which responded more like Desulfovibrio species than the other Desulfotomaculum strains tested. Extracts from colorless or photosynthetic sulfur bacteria were either unreactive or exhibited very low levels of reactivity with the antibodies to the enzyme from sulfate reducers. These results were confirmed by using partially purified enzymes from sulfate reducers and the most cross-reactive sulfide oxidizer, Thiobacillus denitrificans. Two types of monoclonal antibodies to adenosine 5′-phosphosulfate reductase were also isolated. One type reacted more variably with the enzymes of the sulfate reducers and poorly with the Thiobacillus enzyme, whereas the second reacted strongly with Desulfovibrio, Desulfotomaculum orientis, and Thiobacillus enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron-sulfur proteins. Anal Biochem. 1983 Jun;131(2):373–378. doi: 10.1016/0003-2697(83)90186-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
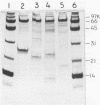
- Bramlett R. N., Peck H. D., Jr Some physical and kinetic properties of adenylyl sulfate reductase from Desulfovibrio vulgaris. J Biol Chem. 1975 Apr 25;250(8):2979–2986. [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. A. Sulphate-reducing bacteria and anaerobic corrosion. Annu Rev Microbiol. 1985;39:195–217. doi: 10.1146/annurev.mi.39.100185.001211. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kennedy M. C., Kent T. A., Emptage M., Merkle H., Beinert H., Münck E. Evidence for the formation of a linear [3Fe-4S] cluster in partially unfolded aconitase. J Biol Chem. 1984 Dec 10;259(23):14463–14471. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. P., Yi C. S., LeGall J., Peck H. D., Jr Isolation of a new pigment, desulforubidin, from Desulfovibrio desulfuricans (Norway strain) and its role in sulfite reduction. J Bacteriol. 1973 Jul;115(1):453–455. doi: 10.1128/jb.115.1.453-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Norqvist A., Roffey R. Biochemical and immunological study of cell envelope proteins in sulfate-reducing bacteria. Appl Environ Microbiol. 1985 Jul;50(1):31–37. doi: 10.1128/aem.50.1.31-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck H. D., Jr Energy-coupling mechanisms in chemolithotrophic bacteria. Annu Rev Microbiol. 1968;22:489–518. doi: 10.1146/annurev.mi.22.100168.002421. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., HANDLER P. THE ABSORPTION SPECTRA OF IRON-FLAVOPROTEINS. J Biol Chem. 1964 May;239:1509–1514. [PubMed] [Google Scholar]
- Schwenn J. D., Krone F. A., Husmann K. Yeast PAPS reductase: properties and requirements of the purified enzyme. Arch Microbiol. 1988;150(4):313–319. doi: 10.1007/BF00408300. [DOI] [PubMed] [Google Scholar]
- Skyring G. W., Trudinger P. A. A comparison of the electrophoretic properties of the ATP-sulfurylases, APS-reductases, and sulfite reductases from cultures of dissimilatory sulfate-reducing bacteria. Can J Microbiol. 1973 Mar;19(3):375–380. doi: 10.1139/m73-061. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., ASAHI T., BANDURSKI R. S. Yeast sulfate-reducing system. I. Reduction of sulfate to sulfite. J Biol Chem. 1961 Jun;236:1822–1829. [PubMed] [Google Scholar]
- Weimer P. J., Van Kavelaar M. J., Michel C. B., Ng T. K. Effect of Phosphate on the Corrosion of Carbon Steel and on the Composition of Corrosion Products in Two-Stage Continuous Cultures of Desulfovibrio desulfuricans. Appl Environ Microbiol. 1988 Feb;54(2):386–396. doi: 10.1128/aem.54.2.386-396.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]