Abstract
Two Pseudomonas spp. (isolates 50552 and 50581) isolated from soil degraded 1-naphthol and carbaryl, an N-methylcarbamate pesticide, respectively. They utilized these compounds as a sole source of carbon. 1-Naphthol was completely metabolized to CO2 by the isolate 50552, while the carbaryl was first hydrolyzed to 1-naphthol and then converted into a brown-colored compound by the isolate 50581. The colored metabolite was not degraded, but 1-naphthol produced by the isolate 50581 during the exponential phase of growth was metabolized by the isolate 50552. The two isolates were used to construct a bacterial consortium which completely catabolized carbaryl to CO2. No metabolite was detected in the cell cultures of the consortium. The isolate 50581 harbored a 50-kb plasmid pCD1, while no plasmid was detected in the isolate 50552. The isolated bacteria individually or as a consortium may be used for detoxification of certain industrial and agricultural wastes.
Full text
PDF

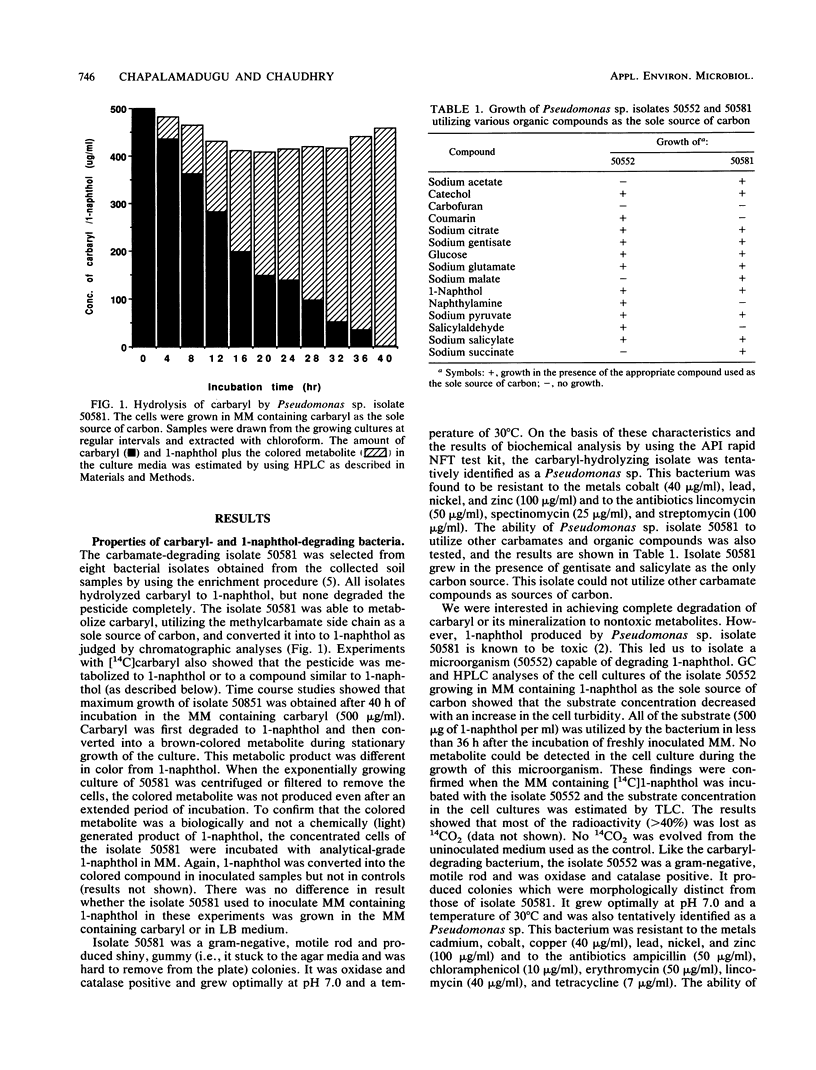
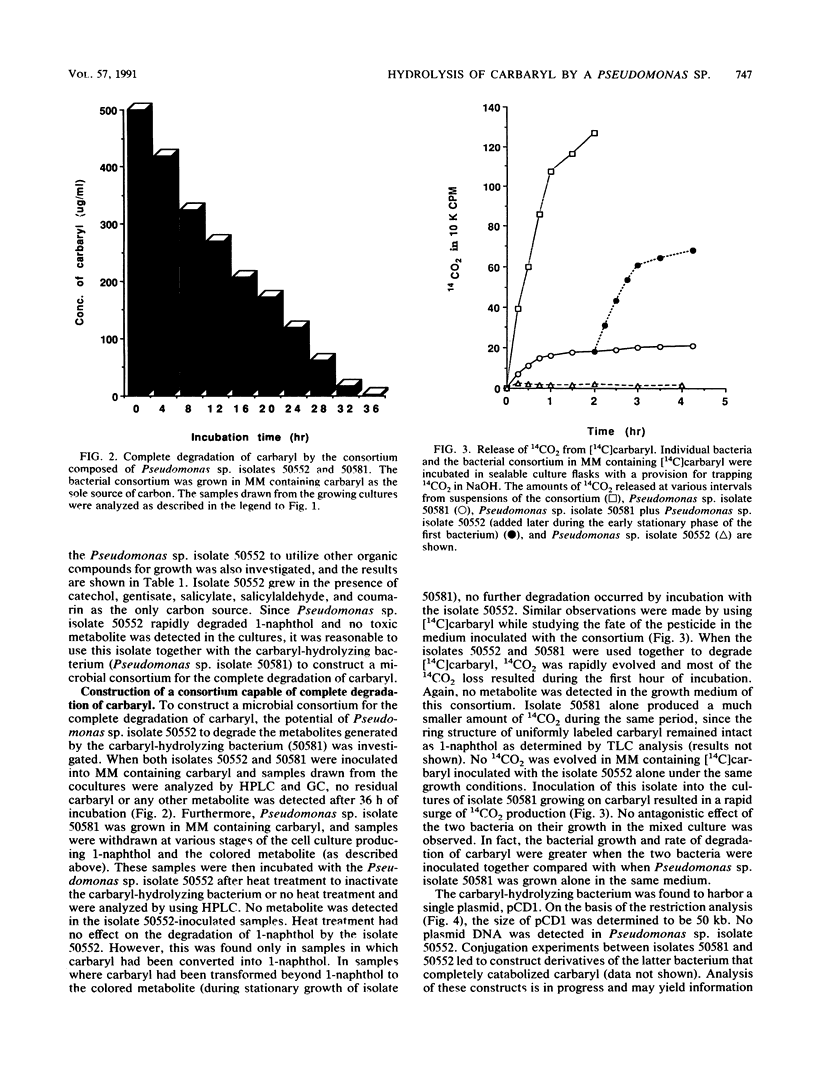

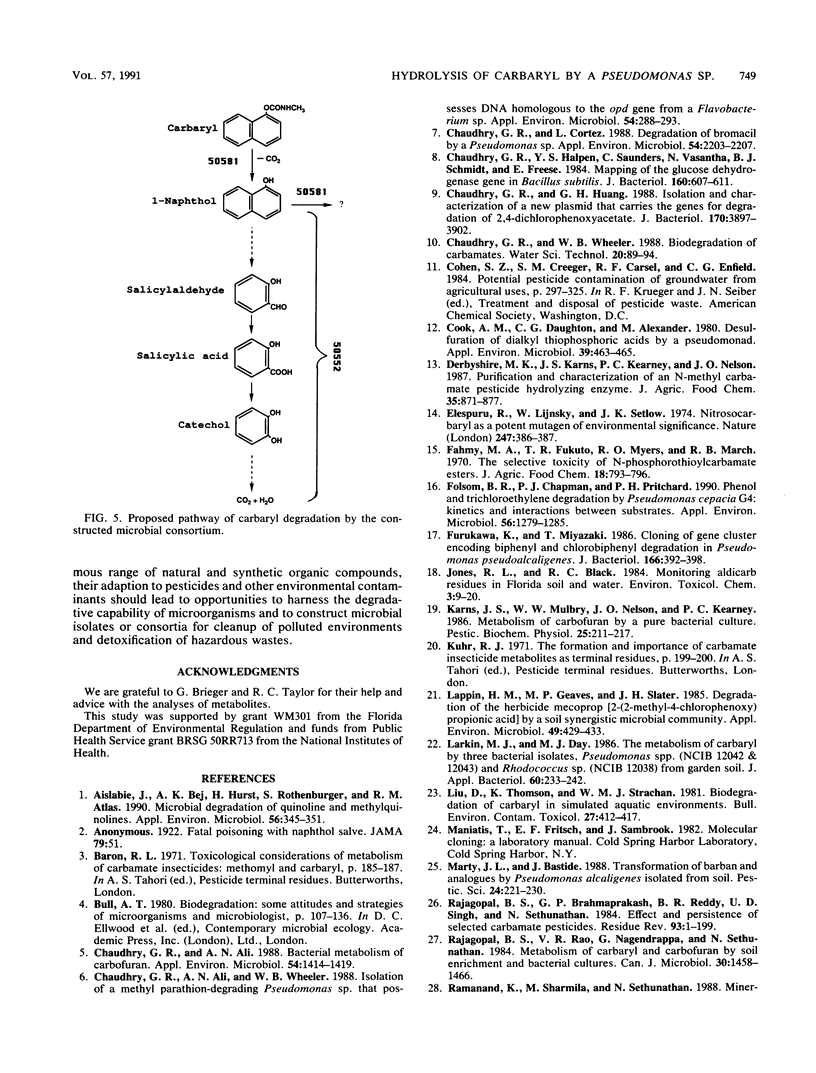

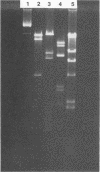
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aislabie J., Bej A. K., Hurst H., Rothenburger S., Atlas R. M. Microbial degradation of quinoline and methylquinolines. Appl Environ Microbiol. 1990 Feb;56(2):345–351. doi: 10.1128/aem.56.2.345-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Ali A. N. Bacterial metabolism of carbofuran. Appl Environ Microbiol. 1988 Jun;54(6):1414–1419. doi: 10.1128/aem.54.6.1414-1419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Ali A. N., Wheeler W. B. Isolation of a methyl parathion-degrading Pseudomonas sp. that possesses DNA homologous to the opd gene from a Flavobacterium sp. Appl Environ Microbiol. 1988 Feb;54(2):288–293. doi: 10.1128/aem.54.2.288-293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Cortez L. Degradation of bromacil by a Pseudomonas sp. Appl Environ Microbiol. 1988 Sep;54(9):2203–2207. doi: 10.1128/aem.54.9.2203-2207.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Halpern Y. S., Saunders C., Vasantha N., Schmidt B. J., Freese E. Mapping of the glucose dehydrogenase gene in Bacillus subtilis. J Bacteriol. 1984 Nov;160(2):607–611. doi: 10.1128/jb.160.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Huang G. H. Isolation and characterization of a new plasmid from a Flavobacterium sp. which carries the genes for degradation of 2,4-dichlorophenoxyacetate. J Bacteriol. 1988 Sep;170(9):3897–3902. doi: 10.1128/jb.170.9.3897-3902.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Daughton C. G., Alexander M. Desulfuration of dialkyl thiophosphoric acids by a pseudomonad. Appl Environ Microbiol. 1980 Feb;39(2):463–465. doi: 10.1128/aem.39.2.463-465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elespuru R., Lijinsky W., Setlow J. K. Nitrosocarbaryl as a potent mutagen of environmental significance. Nature. 1974 Feb 8;247(5440):386–387. doi: 10.1038/247386a0. [DOI] [PubMed] [Google Scholar]
- Fahmy M. A., Fukuto T. R., Myers R. O., March R. B. The selective toxicity of new N-phosphorothioyl-carbamate esters. J Agric Food Chem. 1970 Sep-Oct;18(5):793–796. doi: 10.1021/jf60171a014. [DOI] [PubMed] [Google Scholar]
- Folsom B. R., Chapman P. J., Pritchard P. H. Phenol and trichloroethylene degradation by Pseudomonas cepacia G4: kinetics and interactions between substrates. Appl Environ Microbiol. 1990 May;56(5):1279–1285. doi: 10.1128/aem.56.5.1279-1285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986 May;166(2):392–398. doi: 10.1128/jb.166.2.392-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin H. M., Greaves M. P., Slater J. H. Degradation of the herbicide mecoprop [2-(2-methyl-4-chlorophenoxy)propionic Acid] by a synergistic microbial community. Appl Environ Microbiol. 1985 Feb;49(2):429–433. doi: 10.1128/aem.49.2.429-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. J., Day M. J. The metabolism of carbaryl by three bacterial isolates, Pseudomonas spp. (NCIB 12042 & 12043) and Rhodococcus sp. (NCIB 12038) from garden soil. J Appl Bacteriol. 1986 Mar;60(3):233–242. doi: 10.1111/j.1365-2672.1986.tb01078.x. [DOI] [PubMed] [Google Scholar]
- Liu D., Thomson K., Strachan W. M. Biodegradation of carbaryl in simulated aquatic environment. Bull Environ Contam Toxicol. 1981 Sep;27(3):412–417. doi: 10.1007/BF01611041. [DOI] [PubMed] [Google Scholar]
- Ramanand K., Sharmila M., Sethunathan N. Mineralization of carbofuran by a soil bacterium. Appl Environ Microbiol. 1988 Aug;54(8):2129–2133. doi: 10.1128/aem.54.8.2129-2133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard R. W., Dorough H. W. In vivo formation of nitrosocarbamates in the stomach of rats and guinea pigs. J Toxicol Environ Health. 1984;14(2-3):279–290. doi: 10.1080/15287398409530580. [DOI] [PubMed] [Google Scholar]
- Rodriguez L. D., Dorough H. W. Degradation of carbaryl by soil microorganisms. Arch Environ Contam Toxicol. 1977;6(1):47–56. doi: 10.1007/BF02097748. [DOI] [PubMed] [Google Scholar]
- Shelton D. R. Mineralization of diethylthiophosphoric Acid by an enriched consortium from cattle dip. Appl Environ Microbiol. 1988 Oct;54(10):2572–2573. doi: 10.1128/aem.54.10.2572-2573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek P. H., Karns J. S. Cloning of a carbofuran hydrolase gene from Achromobacter sp. strain WM111 and its expression in gram-negative bacteria. J Bacteriol. 1989 Jul;171(7):4038–4044. doi: 10.1128/jb.171.7.4038-4044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K. M., Gunsalus I. C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki M. H., Moran D., Harris D. Pesticides in groundwater: the aldicarb story in Suffolk County, NY. Am J Public Health. 1982 Dec;72(12):1391–1395. doi: 10.2105/ajph.72.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]