Abstract
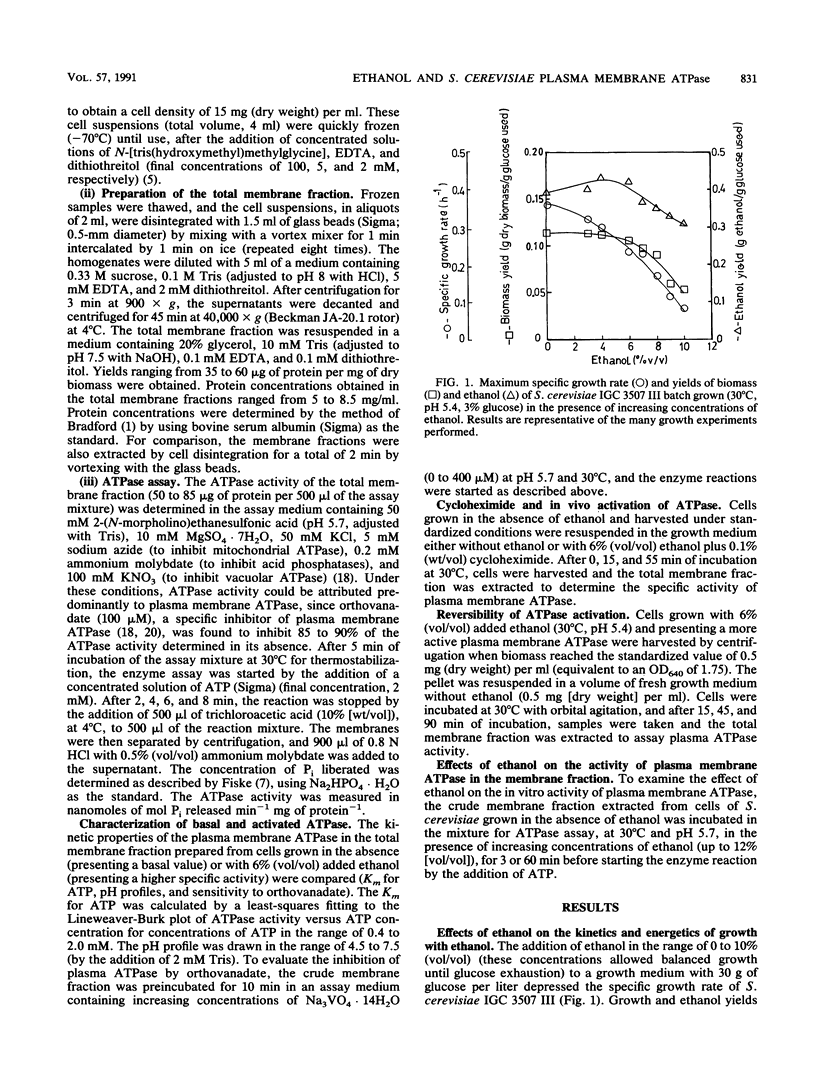
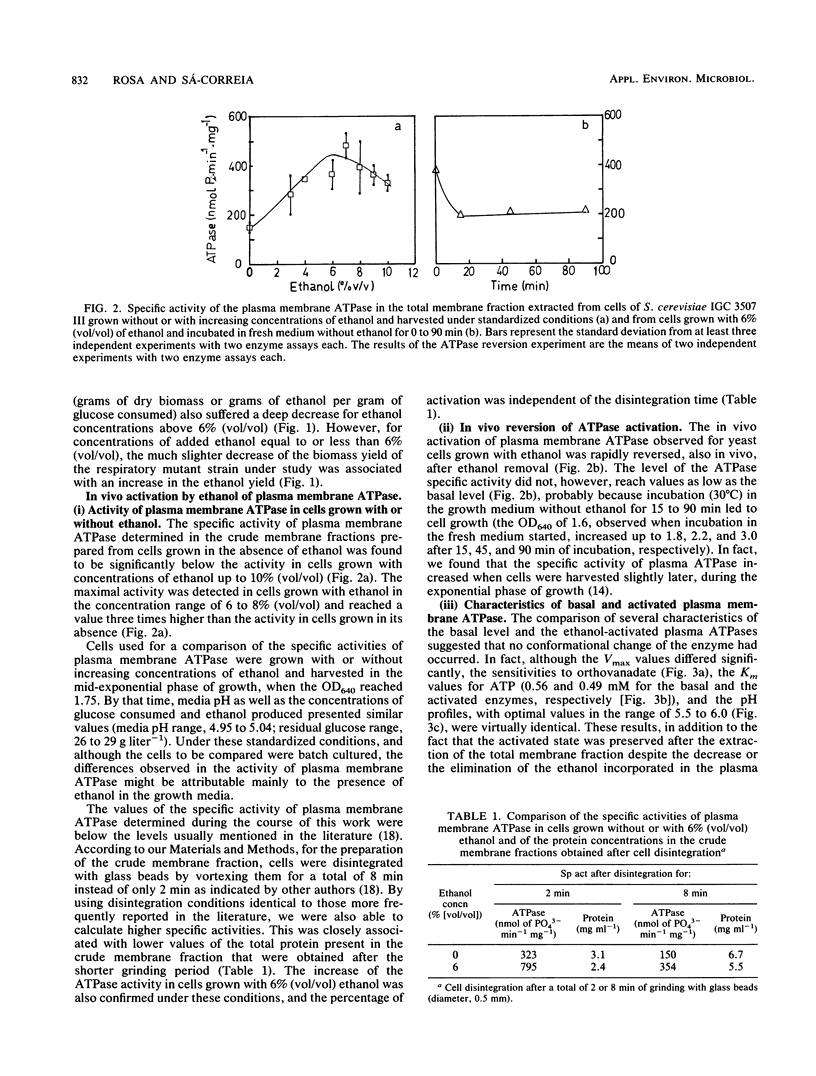
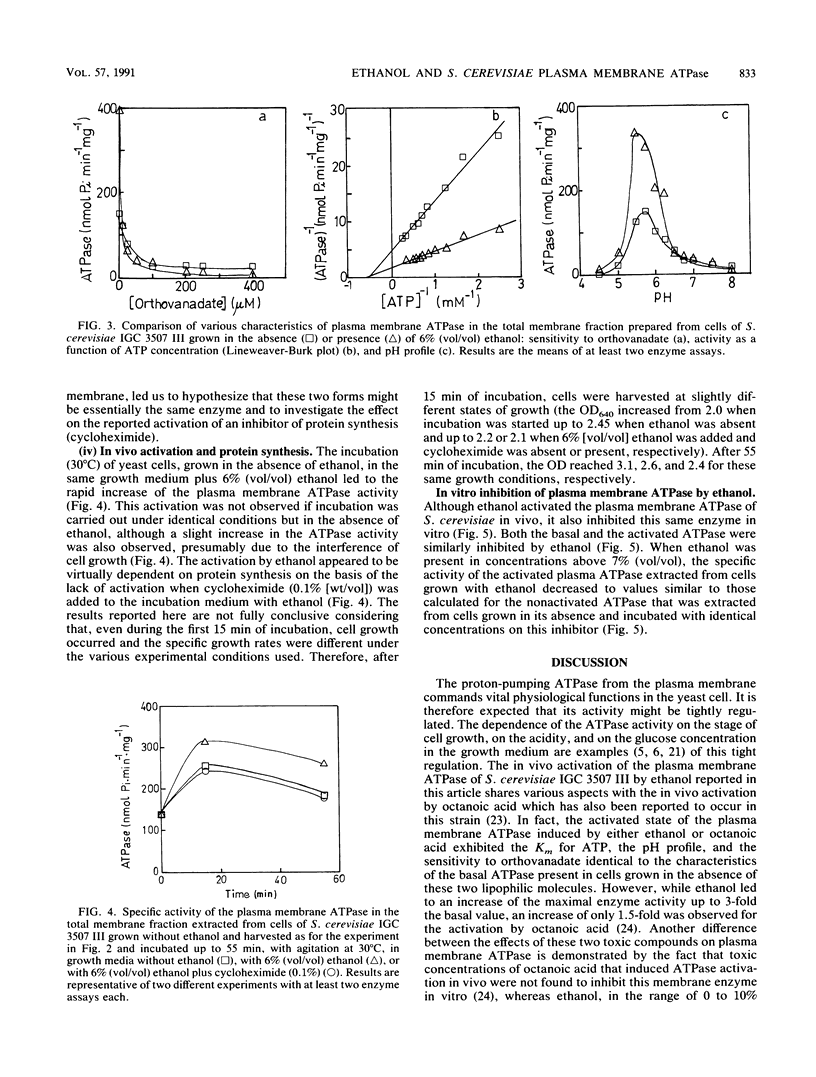
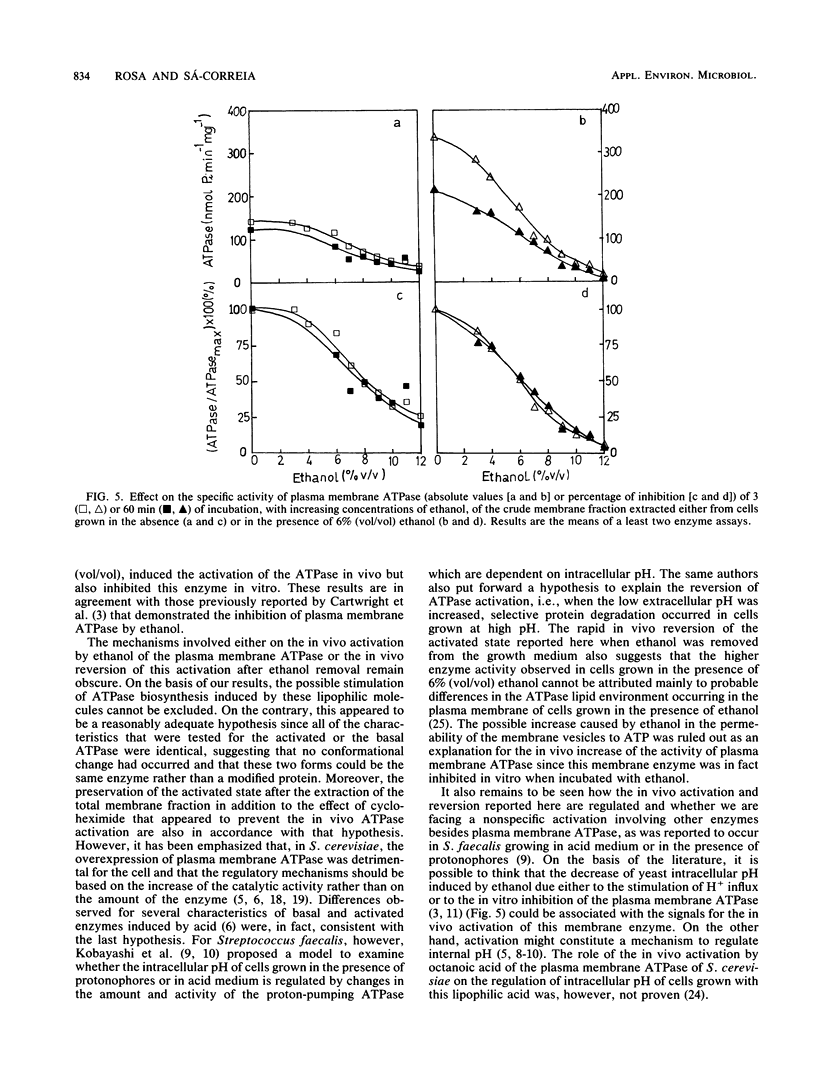
Ethanol, in concentrations that affect growth and fermentation rates (3 to 10% [vol/vol]), activated in vivo the plasma membrane ATPase of Saccharomyces cerevisiae. The maximal value for this activated enzyme in cells grown with 6 to 8% (vol/vol) ethanol was three times higher than the basal level (in cells grown in the absence of ethanol). The Km values for ATP, the pH profiles, and the sensitivities to orthovanadate of the activated and the basal plasma membrane ATPases were virtually identical. A near-equivalent activation was also observed when cells grown in the absence of ethanol were incubated for 15 min in the growth medium with ethanol. The activated state was preserved after the extraction from the cells of the membrane fraction, and cycloheximide appeared to prevent this in vivo activation. After ethanol removal, the rapid in vivo reversion of ATPase activation was observed. While inducing the in vivo activation of plasma membrane ATPase, concentrations of ethanol equal to and greater than 3% (vol/vol) also inhibited this enzyme in vitro. The possible role of the in vivo activation of the plasma membrane proton-pumping ATPase in the development of ethanol tolerance by this fermenting yeast was discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cartwright C. P., Veazey F. J., Rose A. H. Effect of ethanol on activity of the plasma-membrane ATPase in, and accumulation of glycine by, Saccharomyces cerevisiae. J Gen Microbiol. 1987 Apr;133(4):857–865. doi: 10.1099/00221287-133-4-857. [DOI] [PubMed] [Google Scholar]
- Dombek K. M., Ingram L. O. Ethanol production during batch fermentation with Saccharomyces cerevisiae: changes in glycolytic enzymes and internal pH. Appl Environ Microbiol. 1987 Jun;53(6):1286–1291. doi: 10.1128/aem.53.6.1286-1291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraso P., Cid A., Serrano R. Tight control of the amount of yeast plasma membrane ATPase during changes in growth conditions and gene dosage. FEBS Lett. 1987 Nov 16;224(1):193–197. doi: 10.1016/0014-5793(87)80446-5. [DOI] [PubMed] [Google Scholar]
- Eraso P., Gancedo C. Activation of yeast plasma membrane ATPase by acid pH during growth. FEBS Lett. 1987 Nov 16;224(1):187–192. doi: 10.1016/0014-5793(87)80445-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Suzuki T., Kinoshita N., Unemoto T. Amplification of the Streptococcus faecalis proton-translocating ATPase by a decrease in cytoplasmic pH. J Bacteriol. 1984 Jun;158(3):1157–1160. doi: 10.1128/jb.158.3.1157-1160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Suzuki T., Unemoto T. Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J Biol Chem. 1986 Jan 15;261(2):627–630. [PubMed] [Google Scholar]
- Leão C., Van Uden N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Jul 11;774(1):43–48. doi: 10.1016/0005-2736(84)90272-4. [DOI] [PubMed] [Google Scholar]
- Salgueiro S. P., Sá-Correia I., Novais J. M. Ethanol-Induced Leakage in Saccharomyces cerevisiae: Kinetics and Relationship to Yeast Ethanol Tolerance and Alcohol Fermentation Productivity. Appl Environ Microbiol. 1988 Apr;54(4):903–909. doi: 10.1128/aem.54.4.903-909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase of fungi and plants as a novel type of proton pump. Curr Top Cell Regul. 1984;23:87–126. doi: 10.1016/b978-0-12-152823-2.50007-6. [DOI] [PubMed] [Google Scholar]
- Serrano R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta. 1988 Feb 24;947(1):1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- Tuduri P., Nso E., Dufour J. P., Goffeau A. Decrease of the plasma membrane H+-ATPase activity during late exponential growth of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1985 Dec 31;133(3):917–922. doi: 10.1016/0006-291x(85)91223-9. [DOI] [PubMed] [Google Scholar]
- Viegas C. A., Rosa M. F., Sá-Correia I., Novais J. M. Inhibition of Yeast Growth by Octanoic and Decanoic Acids Produced during Ethanolic Fermentation. Appl Environ Microbiol. 1989 Jan;55(1):21–28. doi: 10.1128/aem.55.1.21-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Caprioglio H. M., Casey W. M., Parks L. W. Saccharomyces cerevisiae membrane sterol modifications in response to growth in the presence of ethanol. Appl Environ Microbiol. 1990 Sep;56(9):2853–2857. doi: 10.1128/aem.56.9.2853-2857.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



