Abstract
We identified long-term (up to 12 weeks), bilateral changes in spontaneous and evoked pain behavior and baseline forebrain activity following a chronic constriction injury (CCI) of the sciatic nerve. The long-term changes in basal forebrain activation following CCI were region-specific and can be divided into forebrain structures that showed either: (1) no change, (2) an increase, or (3) a decrease in activity with regard to the short-term (2 weeks) changes we previously reported. All the rats showed spontaneous pain behaviors that persisted throughout the 12-week observation period, resembling the pattern of change found in four limbic system structures: the anterior dorsal thalamus, habenular complex, and the cingulate and retrosplenial cortices. In contrast, heat hyperalgesia was delayed in onset until 4 weeks following CCI, but then persisted, showing a nearly constant level of increased responsiveness. The forebrain activation that resembles this behavioral pattern of change is found in somatosensory cortex, and in the hypothalamic paraventricular nucleus and the basolateral amygdala. Finally, mechanical allodynia, which was maximal during the first 2 weeks following nerve injury and gradually recovered by the seventh post-operative week uniquely matches the time course of changes in ventrolateral and ventroposterolateral thalamic activity. Our results indicate that peripheral nerve damage results in persistent changes in behavior and resting forebrain systems that modulate pain perception. The persistent abnormalities in the somatosensory cortex and thalamus suggest that the sensory thalamocortical axis is functionally deranged in certain chronic pain states.
Keywords: Chronic pain, Chronic constriction injury, Sciatic nerve, Neuroimaging, Regional cerebral blood flow, Rat
1. Introduction
Numerous papers have been published over the last decade using the Bennett and Xie (1988) animal model of chronic neuropathic pain in which loose ligatures are tied around the sciatic nerve. Behaviorally, this model has been very well characterized: animals develop spontaneous pain-related behaviors (abnormal paw postures, scratching, altered walking patterns), allodynia, and hyperalgesia to both thermal and mechanical stimuli (Bennett and Xie, 1988; Kingery and Vallin, 1989; Attal et al., 1990; Kupers et al., 1992; Coggeshall et al., 1993; Ro and Jacobs, 1993; Jacobs and Ro, 1994). These pain syndromes appear to maximize around 2-weeks post-operative with a gradual return to control values by 4–10 weeks post-operative (Bennett and Xie, 1988; Attal et al., 1990; Kupers et al., 1992; Coggeshall et al., 1993; Ro and Jacobs, 1993; Jacobs and Ro, 1994).
In attempts to elucidate the neural mechanisms of chronic pain, researchers have found ectopic discharges generated in the dorsal root ganglia (Kajander et al., 1992; Xie et al., 1995) and at the injury site (Tal and Eliav, 1996). The abnormalities in the activity of dorsal horn cells have been observed: some neurons present spontaneous activity and/or long after-discharges (Hylden et al., 1987; Palecek et al., 1992; Kajander and Bennett, 1992; Laird and Bennett, 1993; Sotgiu et al., 1995). An increase in spinal and supraspinal neuronal activity as estimated by the [14C]2-deoxyglucose technique has also been reported with the chronic constriction injury (CCI) model (Mao et al., 1992a, 1993). The immediate early genes, also used as an indirect marker of neuronal activity show a clear increase in the number of Fos-like immunoreactive neurons at the level of the spinal cord immediately, and up to 10 days after peripheral nerve lesions (Herdegen et al., 1992; Chi et al., 1993; Hirakawa and Kawata, 1993; Hunt et al., 1994; Kajander et al., 1996). At the forebrain level, changes in reactivity have been observed at the thalamus and somatosensory cortex (Guilbaud et al., 1991, 1992). We recently reported that CCI produced bilateral increases in activation within multiple forebrain structures in unstimulated animals (Paulson et al., 2000), using regional cerebral blood flow (rCBF) as an indication of neuronal activity. We found that CCI resulted in increased rCBF in the hind limb (HL) region of somatosensory cortex, as well as multiple thalamic nuclei, including the ventral medial (VM), ventral posterior lateral (VPL), and the posterior nuclear groups. In addition, several fore-brain regions considered part of the limbic system showed pain-induced changes in rCBF, including the anterior dorsal (AD) nucleus of the thalamus, cingulate cortex, retrosplenial cortex, the habenular complex, the interpeduncular nucleus (IPN), and the paraventricular nucleus (PVN) of the hypothalamus.
The aim of the present study was to determine the changes in forebrain rCBF that parallel the changes in behavior over time following CCI. For this study, we looked only at basal rCBF, in the absence of intentional stimulation, in neuropathic rats at select time points after a CCI to the sciatic nerve.
2. Methods
2.1. Animal subjects
Forty-five male, Sprague–Dawley rats weighing between 225 and 350 g served as subjects for these experiments. All the animals were housed individually and maintained on a 12–12 light–dark cycle, with lights on at 06:00 hrs. The ambient temperature in the animal facility was kept at 22 ± 2°C. Food and water were given ad libitum. Our Institutional Animal Care and Use Committee approved all the experimental procedures. We conducted all the experiments in accordance with the guidelines of the National Institutes of Health (NIH) for the ethical use of laboratory animals and of the IASP for use of conscious animals in pain research (Zimmermann, 1983).
2.2. Surgery
Animals were anesthetized with xylazine and ketamine (13 mg/kg intramuscularly (i.m.) and 87 mg/kg i.m., respectively). A CCI was produced by ligating the common sciatic nerve on the left side using the method described by Bennett and Xie (1988). Briefly, the common sciatic nerve was exposed at the level of the middle of the thigh by blunt dissection through the biceps femoris. Proximal to the its trifurcation, about 7 mm of nerve was freed of adhering tissue and three ligatures (using 4.0 Dexon™ braided poly-glycolic acid coated suture) were tied loosely around it at 1-mm intervals. The ligatures just barely constricted the diameter of the nerve when viewed by 30 × magnification. This degree of constriction retarded, but did not arrest, the circulation through the superficial epineural vasculature and produced a small, brief twitch in the muscle around the exposure.
2.3. Groups
Non-ligated controls (N = 11)
For baseline measurement of rCBF in the absence of intentional somatosensory stimulation, each rat was allowed to remain undisturbed prior to, during and for 2–5 min following tracer injection (see below).
Chronic pain groups (N = 34)
For examining changes in rCBF in the absence of intentional somatosensory stimulation 2, 8, or 12 weeks following CCI, we injected each animal with an intravenous (i.v.) bolus of radiotracer. Each rat was then allowed to remain undisturbed prior to, during, and for 2–5 min following tracer injection.
2.4. Quantification of behavior
All the animals were given 1–2 weeks to acclimate to the colony room before undergoing any surgical procedure. Over this period the rats were handled extensively and acclimated to gentle restraint in a soft towel and habituated to the various behavioral testing procedures. We quantified the following three behaviors, in the order presented: (1) resting paw posture; (2) response to thermal stimulation; and (3) response to mechanical stimulation. These behaviors were quantified on 13 separate occasions: baseline (2 days prior to surgery) and once weekly for up to 12 weeks post-operative. The sequence of the behavioral tests was kept the same throughout the test period for all animals. The rationale for the choice of such order was that the least stressful test was done first to minimize the influence of one test on the result of the next.
2.4.1. Spontaneous pain
We tested for the presence of spontaneous pain using a method adapted from Attal et al. (1990) where the resting paw posture of each hind paw of each animal was observed without intervention by the experimenter. Each animal was placed in clear rectangular Plexiglas cage (20 cm wide × 60 cm long × 20 cm high) and allowed to habituate for 15 min. The different positions of the ligated or non-ligated hind paw were rated for the next 48-min period, using a time sampling technique. The paw position was recorded for 2 min (120 s) every 16 min according to the following scale: 0 = paw pressed normally on floor; 1 = paw rests lightly on floor, toes ventroflexed; 2 = only internal edge of paw pressed to floor; 3 = only heel pressed to floor, hind paw in inverted position; 4 = whole paw is elevated; 5 = animal licks paw. Each hind paw was rated three times per test day. The average ratings over successive 16-min periods therefore provided an index of pain intensity for each hind paw of each rat for a 48-min period. A weighted pain score for each hind paw of each animal was calculated by multiplying the average amount of time the rat spent in each category according to the following formula: [(t1 + 2)(t2 + 3)(t3 + 4)(t4 + 5)(t5)/120] (s); where t1, t2, t3, t4, and t5 are the duration (in s) spent in categories 1, 2, 3, 4, and 5, respectively. Therefore, the possible scores range from 0 to 5 representing a continuum in paw posture from normal to abnormal.
2.4.2. Mechanical stimulation
The response to mechanical stimuli was quantified by measuring the number of foot withdrawals to application of a von Frey hair (a series of nylon monofilaments of increasing stiffness that exert defined levels of force as they are pressed to the point where they bend). We used the standard Semmes–Weinstein set of von Frey hairs that are used in neurological and psychophysical testing in humans. Five levels of force ranging from 0.217 to 7.370 g were applied. Each rat was placed in a side-by-side wire-hanging cage with a mesh bottom and allowed to habituate for 10 min. A von Frey hair was applied to the plantar surface of the hind foot until just bent. This procedure was repeated five times during each trial, at a frequency of one stimulus/s. Each trial was repeated three times at approximately 5-min intervals on each hind paw. On a given test day, this procedure was repeated for all von Frey hairs, in ascending order, starting with the weakest. The occurrence of foot withdrawal in each of these three trials was expressed as a percent response frequency: (# of foot withdrawals/5 × 100 = % response frequency).
2.4.3. Thermal stimulation
The response to a thermal stimulus was quantified by recording the withdrawal latency to a radiant heat source, using the Hargreaves device. The rats (4–6 at a time) were placed individually into clear plastic cages (10 × 20 cm2) located on an elevated floor of clear glass (2 mm thick) and given 10 min to habituate. A radiant heat source (halogen projector lamp CXL/CXP, 50 W, 8 V), contained in a movable holder placed beneath the glass floor, was used to apply a thermal stimulus by aiming the beam of light through the glass floor to the heel of each hind paw. The stimulus onset activated a timer that was controlled by a photocell positioned to receive reflected light from the hind paw. The hind paw withdrawal reflex automatically turned off the stimulus and stopped the timer, with a maximum cut-off time of 8 s (for a more complete description of the apparatus, see Hargreaves et al., 1988). The hind paws were tested alternately with 10-min intervals between tests. Three latency measurements were taken for each hind paw in each session and the latency was averaged for each side.
2.5. Measurement of regional cerebral blood flow
At 8 or 12 weeks following CCI surgery, the day of rCBF measurement, we placed each rat in the towel restraint and inserted a flexible 24 gauge i.v. catheter through the skin into the tail vein. A rubber capped injection port was attached to the catheter and flushed with 0.25 ml of saline solution. Each animal was then permitted to rest quietly in the restraint for 30–40 min to recover from the stress induced by tail vein catheterization.
For imaging rCBF, we used the recently published method of Morrow et al. (1998). Briefly, 8–10 mCi of technetium ([99m]Tc) exametazime in a 0.5–1 ml total volume was injected through the tail vein catheter as a bolus over 10–15 s. Two to 5 min following tracer injection, the rat was overdosed with chloral hydrate (300 mg/kg, i.v.), removed from the restraint and decapitated. The brain was removed from the skull and prepared for sectioning by rapid freezing with powdered dry ice. Standard 20-μm coronal frozen sections were cut at −18°C using a Hacker-Bright™ cryostat. Three to four consecutive sections taken at fixed intervals were mounted on glass slides and rapidly desiccated on a hotplate at 60°C. The slides were then arranged in a standard X-ray cassette and an autoradiogram was generated by direct apposition of the tissue to the emulsion side of Kodak BioMax™ MR-1 Imaging film (Kodak Inc., Rochester, NY, USA) for a 1.5–3-h exposure.
The densitometric analysis of autoradiograms was performed using a microcomputer-assisted video imaging densitometer (MCID system, Imaging Research Inc., St. Catherines, Ontario, Canada). The anatomic location of selected regions of interest (ROIs, Table 1) was determined using an approach designed to ensure the accuracy and consistency of structural identification (Morrow et al., 1998). Briefly, for each brain section, we overlaid a matching transparent stereotaxic template, adapted from the stereotaxic atlas of Paxinos and Watson (1986), on the digitized brain images displayed on the video monitor and aligned the images using prominent landmarks. The template served as a guide to sample the ROIs present at a given anterior–posterior level. An index of activation (AI) from individual ROIs was calculated as a percent of average total activity of the entire brain.
Table 1.
Anatomical abbreviations for all sampled regions of interest (ROIs)
| AD, anterior dorsal nucleus (thalamus) | PAG, periaqueductal gray (midbrain) |
| BLA, basal lateral amygdala | PAR, parietal cortex |
| CC, cingulate cortex | PO, posterior group (thalamus) |
| CPU, caudate-putamen | PVN, paraventricular nucleus (hypothalamus) |
| HBC, habenular complex | RS, retrosplenial cortex |
| HIP, hippocampus | VL, ventral lateral nucleus (thalamus) |
| HL, hindlimb area of somatosensory cortex | VM, ventral medial nucleus (thalamus) |
| IPN, interpeduncular nucleus | VPL, ventral posterior lateral nucleus (thalamus) |
| MT, medial thalamus | VPM, ventral posterior medial nucleus (thalamus) |
For the purpose of this study, we limited the ROIs to 18 structures. We sampled structures believed to participate in pain processing and others not considered part of traditional pain pathways. Some ROIs were previously identified in human positron emission tomography (PET) as showing increased blood flow during acute pain due to noxious thermal stimuli (Talbot et al., 1991; Casey et al., 1994). Due to our specific interest in the forebrain, we restricted sampling primarily to cortical and thalamic structures and the brainstem periaqueductal gray (PAG). The spinal cord was not examined.
2.6. Data analysis
2.6.1. Behavioral
Parametric statistics, including repeated measures analyses of variance (ANOVA) and Fisher’s least significant difference tests for follow-up pair-wise comparisons were used to analyze group means for the various behavioral tests. P values <0.05 were considered significant.
Within group comparisons
Each animal was tested repeated before surgery and at multiple time points following CCI. A one-way repeated measure ANOVA was used to compare the pre-surgery to post-surgery values for each group.
Between group comparisons
A repeated measures ANOVA was used to determine group differences in the time course of neuropathic pain as measured with each behavioral test. Follow-up two-way ANOVAs were performed after significant differences were found, comparing each group to every other group.
2.6.2. Regional cerebral blood flow
We computed an average AI within subject for each of the 18 ROIs sampled. The mean AIs for each ROI from all animals in each experimental group were then averaged together to compute within group means. The mean AIs of distinctly bilateral structures were examined for side to side differences in rCBF using a paired t-test (P ≤ 0.05). We also tested for significant differences in activation for each ROI between experimental groups using ANOVA with post hoc t-tests (P ≤ 0.05), corrected for multiple comparisons. All statistical analyses were performed using the software package, SPSS for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
The data from the control and short-term (2 weeks) CCI groups was recently published (Paulson et al., 2000) and is presented here only for the purpose of comparison with the long-term (8 and 12 weeks) CCI groups.
3.1. Long-term behavioral effects of CCI
3.1.1. Spontaneous pain behaviors
The results of the test for spontaneous pain-related behaviors are shown in Fig. 1. Before CCI, all animals rested with both hind paws pressed normally to the floor, reflected by a weighted pain score of zero. One week after CCI, the ligated (left) hind paw showed a significant number of occurrences of abnormal paw posture, characterized primarily by pain behaviors 1 and/or 2: paw rests lightly on floor, toes ventroflexed and/or only internal edge of paw pressed to floor. However, a significant percentage (55%) of the CCI animals also showed pain behaviors 4 and 5 on the ligated hind paw: whole paw is elevated and animal licks paw. By 3 weeks post-operative, the pain related behaviors shown by the ligated hindpaw had significantly decreased compared to the 1- and 2-weeks time points, but remained at levels significantly greater than those recorded during the baseline measurement. In comparison, the non-ligated (right) hind-paw also showed significant occurrences of abnormal paw posture, characterized primarily by pain behaviors 1 and/or 2, but these abnormal postures were not evident until 2-weeks post-operative. The CCI animals continued to show significantly increased occurrences of abnormal paw postures of both hind paws for up to 12 weeks after surgery.
Fig. 1.
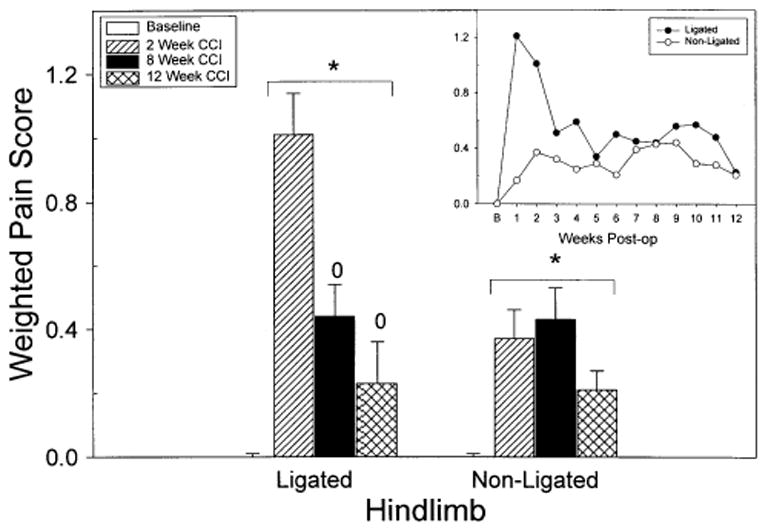
The graphs show the mean weighted pain score for each hind paw of the CCI animals at multiple time points before and after surgery. The weighted pain scores were calculated by multiplying the amount of time the rat spent in each category (see Section 2). The pain condition of the animals at the time of the blood flow experiments (2, 8, and 12 weeks) is shown by the bar graph. The inset (line graph) shows the weighted pain score for all 12 weeks of testing. All the animals were monitored for spontaneous pain behaviors before surgery and received scores of zero. Notice that both hind paws showed a significant increase in spontaneous pain behaviors following surgery. The asterisk (*) indicates a significant increase in the pain score compared to baseline values. The zero (0) indicates a significant difference between the 2-week and the long-term CCI (8 or 12 weeks) animals.
3.1.2. Stimulus-evoked behaviors
Fig. 2 shows the behavioral response made by CCI animals to: (A) mechanical and (B) thermal stimulation in both the ligated and non-ligated hind paw for up to 12 weeks following CCI. Before CCI surgery, the animals responded less than 2% of the time to application of the strongest von Frey stimulation to either hindpaw (panel A). In contrast, by 1 week following CCI, both hind paws showed significant allodynia, evidenced by the 30% (non-ligated) and 40% (ligated) response rate. The response frequency to the 5.07 von Frey monofilament remained significantly greater than pre-CCI for up to 4 and 6 weeks in the right and left hind paws, respectively. The mechanical allodynia was not present in either HL from 7 to 12 weeks post-operative.
Fig. 2.

The mean behavioral response made by CCI animals to (A) mechanical and (B) thermal stimulation when tested before and after CCI surgery. The top panel (A) represents the average percentage of responses made by CCI animals to application of a 5.07 von Frey hair. The percent response was calculated by dividing the number of foot withdrawals elicited by the von Frey hair by the maximum number of responses possible in that trial (see Section 2). The bottom panel (B) represents the average withdrawal latency to a radiant heat source (see Section 2). The CCI produced mechanical allodynia and thermal hyperalgesia in both hind paws. The asterisk (*) indicates a significant difference in the behavioral response compared to baseline values.
The time course of the development of thermal hyperalgesia is presented in Fig. 2 (panel B). Both the ligated and the non-ligated hind paws showed a similar pattern of hyperalgesia over time. The withdrawal latency to the thermal stimulus did not change significantly from baseline for up to 4 weeks following CCI surgery. Notice, however, that at 4 weeks post-operative, both HLs showed robust hyperalgesia that persisted for the entire remaining testing period of up to 12 weeks post-operative. There was no difference in the withdrawal latency between the ligated or non-ligated hindpaw at any time following CCI.
3.2. Long-term effects of CCI on baseline (unstimulated) regional cerebral blood flow
Neither the control nor the long-term CCI groups showed any significant side to side differences in rCBF (data not shown). Because lateralized differences were absent in ligated and control groups, we averaged the AI from both sides for each ROI in all animals before computing group means. All the subsequent analysis and presentation were performed with this pooled data.
The long-term effects of CCI on basal rCBF in multiple forebrain structures are presented in Table 2 and Figs. 3–5. For the purposes of comparison, previously published data from control and 2-week CCI are also presented (Paulson et al., 2000). Because the long-term changes in basal forebrain activity following CCI were very region-specific, we divided forebrain structures into three groups of that showed either: (1) no change, (2) an increase, or (3) a decrease in activity compared to the short-term changes we previously reported at 2 weeks.
Table 2.
Regional differences in cerebral blood flow (rCBF) expressed as the mean percent difference from mean whole brain activity, the activation index (AI)a
| Region of interest | Control (n = 11) | 2-weeks CCI (n = 14) | 8-weeks CCI (n = 12) | 12-weeks CCI (n = 8) |
|---|---|---|---|---|
| AD | 16.29 ± 2.50 | 24.8 ± 2.89 | 30.56 ± 3.70 | 30.98 ± 3.30 |
| BLA | − 1.85 ± 1.19 | 1.46 ± 3.35 | 3.11 ± 2.74 | 11.82 ± 3.53 |
| CC | 8.98 ± 3.10 | 19.33 ± 3.10 | 20.96 ± 3.61 | 15.39 ± 3.52 |
| CPU | 11.52 ± 3.23 | 8.21 ± 4.86 | 7.76 ± 1.83 | 7.29 ± 2.41 |
| HBC | 41.51 ± 5.41 | 51.43 ± 4.92 | 50.17 ± 6.94 | 56.14 ± 3.00 |
| HIP | − 3.68 ± 3.61 | − 5.15 ± 2.07 | − 9.24 ± 2.78 | − 7.11 ± 2.33 |
| HL | 12.36 ± 1.36 | 22.14 ± 2.72 | 35.21 ± 3.91 | 38.40 ± 4.01 |
| IPN | 29.89 ± 7.20 | 41.49 ± 4.18 | 39.65 ± 10.58 | 42.11 ± 9.81 |
| MT | 6.83 ± 3.80 | 8.76 ± 2.26 | 4.18 ± 3.41 | 9.84 ± 3.27 |
| PAG | − 4.76 ± 3.39 | − 6.84 ± 3.39 | − 2.20 ± 9.72 | 6.40 ± 6.47 |
| PAR | 15.81 ± 3.09 | 16.08 ± 2.29 | 31.99 ± 3.02 | 33.21 ± 3.37 |
| PO | 4.23 ± 2.06 | 8.23 ± 3.72 | − 0.19 ± 4.16 | 6.11 ± 4.03 |
| PVN | 9.30 ± 6.48 | 31.65 ± 5.96 | 32.26 ± 6.38 | 54.99 ± 5.74 |
| RS | 21.12 ± 1.97 | 30.23 ± 3.60 | 33.17 ± 4.29 | 31.68 ± 2.80 |
| VL | 4.83 ± 1.84 | 8.24 ± 3.83 | 1.21 ± 4.57 | − 0.97 ± 2.81* |
| VM | 3.66 ± 2.17 | 9.07 ± 3.62 | 1.17 ± 4.04 | − 0.03 ± 3.45 |
| VPL | 4.28 ± 1.74 | 12.48 ± 3.67 | 0.78 ± 3.78 | 0.98 ± 2.98 |
| VPM | 14.51 ± 2.56 | 18.25 ± 3.63 | 16.42 ± 5.57 | 12.59 ± 2.37 |
Values calculated as group means (see Section 2) ± SEM for all regions of interest (ROIs). Bold values, significant difference between CCI and control animals (P ≤ 0.05, ANOVA); underlined values, significant difference from 2-weeks CCI animals (P ≤ 0.05, ANOVA); italicized values, significant difference from 8-weeks CCI animals (P ≤ 0.05, ANOVA);
, indicates that the difference is significant only with a one-tailed t-test.
Fig. 3.

The bar graph compares the average bilateral level of activation in four limbic ROIs for unstimulated control and unstimulated CCI groups, 2, 8, and 12 weeks after surgery. The relative levels of activation are expressed as AI, the mean percent difference from total brain activity (see Section 2). The data are shown only for ROIs showing a significant difference in activation between these groups. The asterisk (*) indicates a significant increase in activation compared to the control value.
Fig. 5.
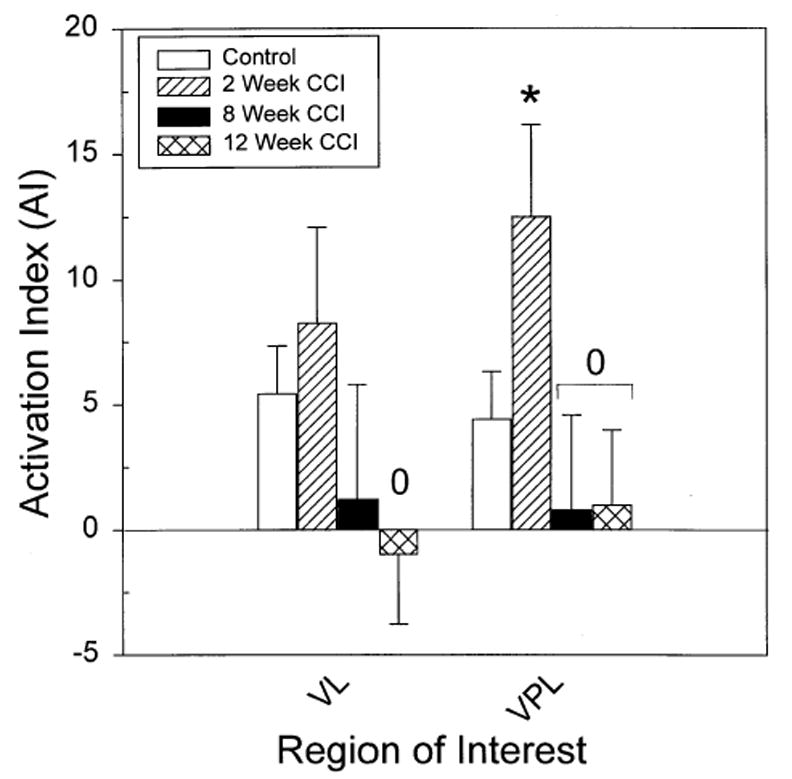
The bar graph compares the average bilateral level of activation in four ROIs for unstimulated control and unstimulated CCI groups, 2, 8, and 12 weeks after surgery. The relative levels of activation are expressed as AI, the mean percent difference from total brain activity (see Section 2). The asterisk (*) indicates a significant increase in activation compared to the control value. The zero (0) indicates a significant difference from the 8- or 12-week CCI animals.
Fig. 3 shows average level of resting forebrain activity in four limbic structures that fit into category 1: no change from the increased blood flow found in the short-term 2-week CCI group. The blood flow in these four limbic structures, specifically, the AD thalamus, habenular complex, and the cingulate and retrosplenial cortices, increased above control values 2 weeks post-operative and remained above control levels for 12 weeks. Note also, that the IPN, which showed a significant increase in blood flow at 2 weeks, remained elevated at 8 and 12 weeks after CCI compared to control values, but the increases were no longer significant (Table 2).
The category 2 long-term effects of CCI on basal rCBF (a significant, further increase compared to the 2-week CCI group) can be seen in Table 2 and Fig. 4. The somatosensory cortex; specifically, the HL and parietal (PAR) areas, showed significant increases in blood flow at 8 and 12 weeks following CCI surgery compared to both the control and 2-weeks groups. Two additional limbic structures, the hypothalamic PVN and the basolateral amygdala (BLA) showed significant increase in blood flow after 8 or 12 weeks following CCI surgery. With the exception of the BLA, all the structures named also showed significant increase in blood flow at 2 weeks after CCI.
Fig. 4.

The bar graph compares the average bilateral level of activation in the VL and VPL thalamic nuclei for unstimulated control and unstimulated CCI groups, 2, 8, and 12 weeks after surgery. The relative levels of activation are expressed as AI, the mean percent difference from total brain activity (see Section 2). The asterisk (*) indicates a significant increase in activation compared to the control value. The zero (0) indicates a significant difference between the 2-week and the long-term CCI (8 or 12 weeks) animals.
In contrast, significant decreases in basal rCBF (category 3) were found in thalamic nuclei at 8 and 12 weeks after CCI when compared to the 2-weeks group (Fig. 5). Specifically, the VPL thalamus, which had initially shown a significant increase in basal blood flow, decreased significantly by 8 and 12 weeks after CCI compared to the 2-weeks CCI group. A similar pattern of change in blood flow, increase followed by decrease, were found in other thalamic nuclei (PO, VL, VM), although only the decrease in rCBF in the ventral lateral (VL) thalamic nuclei at 12 weeks following CCI surgery was significant (one-tailed test) when compared to 2-weeks CCI. None of the long-term CCI groups showed significant changes in resting rCBF in the ventral posteromedial or medial thalamic as compared to the controls.
4. Discussion
This is the first study to use rCBF in an animal model to identify long-term changes in supraspinal activation following CCI. We identified long-term (up to 12 weeks) changes in spontaneous and evoked pain behaviors and baseline forebrain activity following a CCI of the sciatic nerve using an autoradiographic estimate of rCBF. The ligation of the sciatic nerve produced persistent bilateral changes in basal rCBF within multiple forebrain structures associated with somatosensory processing or limbic system function.
All the rats showed behavioral evidence for mechanical allodynia (Fig. 2A), which was bilateral, maximal during the first 2 weeks following nerve injury, and gradually recovered by the seventh post-operative week. This behavioral pattern uniquely matches the time course of changes in baseline VL and VPL thalamic activity (Fig. 5). No other supraspinal structures showed this pattern of recovery. The decrease in basal activity in the thalamus is not unexpected, as thalamic dysfunction has long been thought to play a role in chronic pain. Clinical studies have reported spontaneous pain and disturbed sensations produced by thalamic lesions (Riddoch, 1938; Garcin et al., 1968) and several recent PET studies have demonstrated that there is reduced thalamic rCBF and/or regional glucose metabolism in patients with chronic pain (Canavero et al., 1993; Hirato et al., 1993, 1994; Iadarola et al., 1995; Hsieh et al., 1995). It is possible that thalamic rCBF is down because of reduced inhibitory background activity, but this cannot be proved based on this study. The evidence for this idea is found in the demonstration of increased excitability in the human thalamus with reduced rCBF at rest in central neuropathic pain (Casey, 2000). In addition, results from animal experiments have also shown changes in neuronal responses in thalamic and cortical somatosensory nuclei following nerve injury (Guilbaud et al., 1991, 1992; Rasmusson et al., 1993; Calford and Tweedale, 1991). These observations show that chronic neuropathic pain may be associated with changes in background (unstimulated) thalamic synaptic activity and suggest an important role of the thalamus in pain modulation in addition to that of nociceptive transmission.
In contrast, spontaneous pain behaviors persisted throughout the 12-weeks observation period. The abnormalities were also bilateral, but the spontaneous pain behavior was strongly predominant on the ligated side and showed an incomplete gradual recovery with a plateau at 5–12 weeks following nerve injury. The forebrain activity changes that resemble this behavioral change most closely are found in limbic system structures: the AD thalamus, habenular complex, and the cingulate and retrosplenial cortices (Fig. 3). The activity in each of these structures increased 2 weeks post-operative and maintained a similar level of activity at 12 weeks. The involvement of limbic areas in pain could reflect the evidence of alternative pathways for nociception as provided by anatomical and physiological studies showing direct spinal projections to various limbic areas (Burnstein et al., 1987; Geisler et al., 1994). Such projections can transmit nociceptive information to the limbic areas, either directly (Burnstein et al., 1987; Cliffer et al., 1991), or after a relay in the brainstem (Bernard and Besson, 1990). The areas within the limbic system are thought to play a role in motivational or emotional aspects of behavior, including learning and memory (Melzack and Casey, 1968; Gabriel et al., 1980a,b, 1983; Bouckoms, 1994). The persistent increase in activation in these forebrain structures suggests that central reorganization occurs immediately following CCI and triggers long-term, perhaps permanent, circuit changes within limbic systems.
Unlike all other behaviors, heat hyperalgesia was delayed in onset until 4 weeks following nerve injury. Thereafter, this abnormality persisted in both HLs, showing a nearly constant level of increased responsiveness. This delayed development of hyperalgesia to a radiant heat stimulus differs from other reports using the Hargreaves device in CCI animals (Bennett and Xie, 1988; Kupers et al., 1992; Ro et al., 1999). The reason for this discrepancy is unclear, but may be the result of slightly different methods of stimulation by different experimenters, or from surgical variables. One surgical variable, the anesthetic, differs in our study. We used ketamine in combination with xylazine and the other studies used sodium pentobarbital, isoflurane, hypnorm, or midazolam. Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist and intrathecal injection of competitive or non-competitive NMDA receptor antagonists have been shown to reduce or block the development of thermal hyperalgesia due to CCI (Yamamoto and Yaksh, 1992; Tal and Bennett, 1993, 1994; Mao et al., 1992b,c). Although the effect of NMDA receptor antagonists on the development of thermal hyperalgesia is interesting, the present study was not designed to address this question.
However, it is interesting to note that four structures, the PVN of the hypothalamus, the BLA, and somatosensory cortex (HL and PAR areas) reflect this delayed pattern of behavioral change. The PVN is the principal seat of both magno- and parvicellular neurosecretory neurons, suggesting that this nucleus plays a major role in the coordination of neuroendocrine and autonomic responses (Rho and Swanson, 1987). For example, exposure of animals to immobilization markedly and rapidly increases rates of synthesis, release and metabolism of PVN norepinephrine and leads to the release of corticotropin-releasing hormone (CRF) (Pacak et al., 1995). The analgesic properties of CRF have been demonstrated in many studies (see Lariviere and Melzack, 2000 for review). The CRF has effects on the tonic electrophysiological activity of the locus coeruleus, which is involved in the tonic descending inhibitory control of spinal cord circuits (Valentino and Foote, 1988; Borsody and Weiss, 1996). The CRF also has excitatory actions in the amygdala, cortex and the hypothalamus (Ehlers et al., 1983; Siggins et al., 1985); structures known to be involved in pain processing (Melzack and Wall, 1996). Finally, CRF has predominantly inhibitory actions on neuronal activity in the thalamus, perhaps explaining our findings in the thalamus (Siggins et al., 1985). Although CRF acts at all levels of the neuroaxis, the analgesic effects of CRF appear to be more prominent in tonic, compared to phasic, pain (Lariviere and Melzack, 2000), suggesting that there may be a compensatory increase in CRF during prolonged pain.
The somatosensory cortex (HL and PAR areas) also showed robust increases in activation at 8 and 12 weeks following CCI. Kenshalo and Willis (1991) and Treede et al. (1999) have recently reviewed the evidence for a role of the cerebral cortex in the perception and control of pain. The numerous PET studies have revealed activity in the human sensorimotor cortex during pain (for reviews, see Bushnell et al., 1999; Casey, 1999). We know comparatively less of the functional role of the rodent sensorimotor cortex in nociceptive processing. However, there is strong evidence that the nociceptive responses of neurons in the rat sensorimotor cortex are increased during chronically painful conditions and undergo plastic adaptations (for review, see Besson et al., 1995). Because of the strong reciprocal thalamocortical connections, it is possible that the increased somatosensory cortical activity is directly related to the reduced thalamic activity we observed, and that these changes represent long-term adaptive responses to the ongoing nociceptive activity associated with tissue damage. This would be in general accord with the proposal of Canavero (1994) and Canavero and Bonicalzi (1998), that the sensory thalamocortical axis is functionally deranged in certain chronic pain states.
It is well established that peripheral nerve damage causes major alterations in physiology of the somatosensory system at the spinal, thalamic, and cortical levels (Devor and Wall, 1981a,b; Hylden et al., 1989; Hu and Sessle, 1989; Calford and Tweedale, 1988; Mao et al., 1993; Guilbaud et al., 1991, 1992; Halligan et al., 1993; Rasmusson et al., 1993; Elbert et al., 1994; Salimi et al., 1994). We recently reported increased resting (no overt stimulation) forebrain activity in somatosensory and limbic structures in CCI animals exhibiting spontaneous pain and hyperalgesia 2 weeks after surgery (Paulson et al., 2000). The present results extend those findings and show that peripheral nerve damage results in persistent, and perhaps permanent, changes in behavior and resting forebrain systems that modulate pain perception.
Acknowledgments
This work was supported by the Department of Veterans Affairs, the National Institutes of Health (PO1-HD33986), and the Veterans Education and Research Association of Michigan. We thank Dr Milton Gross and the staff of the Nuclear Medicine Laboratory at the Ann Arbor VAMC for preparing the radiotracer kits used in these experiments. We also express our gratitude to Mediphysics Inc., Arlington Heights, IL, USA (a division of Amersham Inc.) for their generous donation of the Ceretec™ radiotracer kits that were used in this study.
References
- Attal N, Jazat F, Guilbaud G. Further evidence for pain-related behaviors in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in the rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spinol (trigemino) pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Besson J-M, Guilbaud G, Ollat H. Frontiers in pain research. Paris: John Libbey Eurotext; 1995. Forebrain areas involved in pain processing. [Google Scholar]
- Borsody MK, Weiss JM. Influence of corticotropin-releasing hormone on the electrophysiological activity of locus coeruleus neurons. Brain Res. 1996;724:149–168. doi: 10.1016/0006-8993(96)00199-0. [DOI] [PubMed] [Google Scholar]
- Bouckoms AJ. Limbic surgery for pain. In: Wall PD, Melzack R, editors. Textbook of pain. 3. Edinburgh: Churchill Livingstone; 1994. pp. 1171–1187. [Google Scholar]
- Burnstein R, Cliffer KD, Giesler GJ., Jr Direct somatosensory projections from the spinal cord to the hypothalamus and telencephalon. J Neurosci. 1987;7:4159–4164. doi: 10.1523/JNEUROSCI.07-12-04159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA. 1999;96(14):7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford BC, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature (Lond) 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervation. Somatosens Mot Res. 1991;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Canavero S. Dynamic reverberation. A unified mechanism for central and phantom pain. Med Hypotheses. 1994;42:203–207. doi: 10.1016/0306-9877(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Canavero S, Bonicalzi V. The neurochemistry of central pain: evidence from clinical studies, hypothesis and therapeutic implications. Pain. 1998;74(2–3):109–114. doi: 10.1016/s0304-3959(97)00089-4. [DOI] [PubMed] [Google Scholar]
- Canavero S, Pagni CA, Castellano G, Bonicalzi V, Bello M, Duca S, Podio V. The role of cortex in central pain syndromes: preliminary results of a long-term technetium-99 hexamethylpropyleneamineoxime single positron emission computed tomography study. Neurosurgery. 1993;32:185–191. doi: 10.1227/00006123-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Casey KL. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci USA. 1999;96(14):7668–7674. doi: 10.1073/pnas.96.14.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL. Concepts of pain mechanisms: the contribution of functional imaging of the human brain. In: Sandkühler J, Bromm B, Gebhart GF, editors. Nervous system plasticity and chronic pain progress in brain research. Amsterdam: Elsevier; 2000. (chap. 19) [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- Chi SI, Levine JD, Bausbaum AI. Effects of injury discharge on the pesistent expression of spinal cord fos-like immunoreactivity produced by sciatic nerve transection in the rat. Brain Res. 1993;617:220–224. doi: 10.1016/0006-8993(93)91089-b. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Burnstein R, Geisler GJ., Jr Distributions of spinothalamic, spinohypothalamic and spinotelencephalic fibers revealed by antero-grade transport of PHA-L in rats. J Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Dougherty PM, Pover CM, Carlton SM. Is large myelinated fiber loss associated with hyperalgesia in a model of experimental peripheral neuropathy in the rat. Pain. 1993;52:233–242. doi: 10.1016/0304-3959(93)90136-D. [DOI] [PubMed] [Google Scholar]
- Devor M, Wall PD. The effect of peripheral nerve injury on receptive fields of cells in the cat spinal cord. J Comp Neurol. 1981a;199(2):277–291. doi: 10.1002/cne.901990209. [DOI] [PubMed] [Google Scholar]
- Devor M, Wall PD. Plasticity in the spinal cord sensory map following peripheral nerve injury in rats. J Neurosci. 1981b;1(7):679–684. doi: 10.1523/JNEUROSCI.01-07-00679.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in the rat. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. NeuroReport. 1994;5(18):2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Orona E, Foster K, Lambert RW. Cingulate cortical and anterior thalamic neuronal correlates of reversal learning in rabbits. J Comp Physiol Psychol. 1980a;94(6):1087–1100. doi: 10.1037/h0077739. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Foster K, Orona E. Interaction of laminae of the cingulate cortex with the anteroventral thalamus during behavioral learning. Science. 1980b;208(4447):1050–1052. doi: 10.1126/science.7375917. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR. Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behav Neurosci. 1983;97(5):675–696. doi: 10.1037//0735-7044.97.5.675. [DOI] [PubMed] [Google Scholar]
- Garcin R. Thalamic syndrome and pain of central origin. In: Soulairac A, Cahn J, Charpentier J, editors. Pain. London: Academic Press; 1968. pp. 521–541. [Google Scholar]
- Geisler GJ, Jr, Katter JT, Dado RJ. Direct spinal pathways to the limbic system for nociceptive information. Trends Neurosci. 1994;17:244–250. doi: 10.1016/0166-2236(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Benoist JM, Jazat F, Gauton M. Neuronal responsiveness in the ventrobasal thalamic complex of rats with an experimental peripheral mononeuropathy. J Physiol. 1991;64:1537–1554. doi: 10.1152/jn.1990.64.5.1537. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Benoist JM, Levante A, Gautron M, Willer JC. Primary somatosensory cortex in rats with pain-related behaviors due to a peripheral mononeuropathy after moderate ligation of one sciatic nerve: neuronal responsivity to somatic stimulation. Exp Brain Res. 1992;92:227–245. doi: 10.1007/BF00227967. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Marshall JC, Wade DT, Davey J, Morrison D. Thumb in cheek? Sensory reorganization and perceptual plasticity after limb amputation. NeuroReport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Fiallos-Estrada CE, Schmid W, Bravo R, Zimmerman M. The transcription factors c-Jun, Jun D and CREB but not Fos and KROX-24, are differentially regulated in axotomized neurons following transection of rat sciatic nerve. Mol Brain Res. 1992;14:155–165. doi: 10.1016/0169-328x(92)90170-g. [DOI] [PubMed] [Google Scholar]
- Hirakawa M, Kawata M. Distribution pattern of c-Fos expression induced by sciatic nerve sectioning in the rat central nervous system. Journal fur Hirnforschung. 1993;34(3):431–444. [PubMed] [Google Scholar]
- Hirato M, Horikoshi S, Kawashima Y, Satake K, Shibasaki T, Ohye C. The possible role of the cerebral cortex adjacent to the central sulcus for the genesis of central (thalamic) pain – a metabolic study. Acta Neurochir. 1993;58:141–144. doi: 10.1007/978-3-7091-9297-9_32. Suppl. [DOI] [PubMed] [Google Scholar]
- Hirato M, Watanabe K, Takahashi A, Hayase N, Horikoshi S, Shibasaki T, Ohye C. Pathophysiology of central (thalamic) pain: combined change of sensory thalamus with cerebral cortex around central sulcus. Stereo-tact Funct Neurosurg. 1994;62(1–4):300–303. doi: 10.1159/000098636. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- Hu JW, Sessle BJ. Effects of tooth pulp deafferentation on nociceptive and nonnociceptive neurons of the feline trigeminal subnucleus caudalis (medullary dorsal horn) J Neurophys. 1989;61(6):1197–1206. doi: 10.1152/jn.1989.61.6.1197. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Smith GB, Bond A, Munglani R, Thomas K, Elliot PJ, editors. Neuropeptides, nociception and pain. London: Chapman & Hall; 1994. Changes in immediate early gene expression, neuropeptide immunoreactivity and other neuronal markers in the spinal cord and dorsal root ganglion in a rat model of mononeuropathy; pp. 329–349. [Google Scholar]
- Hylden JL, Nahin RL, Dubner R. Altered responses of nociceptive cat lamina I spinal dorsal horn neurons after chronic sciatic neuroma formation. Brain Research. 1987;411(2):341–350. doi: 10.1016/0006-8993(87)91086-9. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37:229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Jacobs JM, Ro LS. A morphological study of experimental mononeuropathy in the rat: early ischemic changes. Journal of the Neurological Sciences. 1994;127(2):143–152. doi: 10.1016/0022-510x(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63:55–64. doi: 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Madsen AM, Iadarola MJ, Draisci G, Wakisaka S. Fos-like immunoreactivity increases in the lumbar spinal cord following a chronic constriction injury to the sciatic nerve of the rat. Neurosci Lett. 1996;206:9–12. doi: 10.1016/0304-3940(96)12447-2. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Willis WD. The role of the cerebral cortex in pain sensation. In: Peters A, editor. Cerebral cortex. Vol. 9. New York, NY: Plenum Press; 1991. pp. 153–212. [Google Scholar]
- Kingery WS, Vallin JA. The development of chronic mechanical hyperalgesia, autotomy and collateral sprouting following sciatic nerve section in rat. Pain. 1989;38(3):321–332. doi: 10.1016/0304-3959(89)90219-4. [DOI] [PubMed] [Google Scholar]
- Kupers RC, Nuytten D, De Castro-Costa M, Gybels JM. A time course analysis of the changes in spontaneous and evoked behaviour in a rat model of neuropathic pain. Pain. 1992;50:101–111. doi: 10.1016/0304-3959(92)90117-T. [DOI] [PubMed] [Google Scholar]
- Laird JMA, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental perioheral neuropathy. J Neurophysiol. 1993;69:2072–2085. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84(1):1–12. doi: 10.1016/S0304-3959(99)00193-1. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Coghill RC, Mayer DJ, Hayes RL. Spatial patterns of spinal cord [14C]-2-deoxyglucose metabolic activity in a rat model of painful peripheral mononeuropathy. Pain. 1992a;50:89–100. doi: 10.1016/0304-3959(92)90116-S. (published erratum appears in Pain 1992;51(3):389) [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992b;598:271–278. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ, Lu J, Hayes RL. Intrathecal MK-801 and local nerve anesthesia synergistically reduce nociceptive behaviors in rats with experimental peripheral mononeuropathy. Brain Res. 1992c;576:254–262. doi: 10.1016/0006-8993(92)90688-6. [DOI] [PubMed] [Google Scholar]
- Mao J, Mayer DJ, Price DD. Patterns of increased brain activity indicative of pain in a rat model of peripheral mononeuropathy. J Neurosci. 1993;13:2689–2702. doi: 10.1523/JNEUROSCI.13-06-02689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain: a new conceptual model. In: Kenshalo DR, editor. The skin senses. Springfield, IL: C.C. Thomas; 1968. pp. 423–443. [Google Scholar]
- Melzack R, Wall PD. The challenge of pain. 2. London: Penguin; 1996. p. 339. [Google Scholar]
- Morrow TJ, Paulson PE, Danneman PJ, Casey KL. Regional changes in forebrain activation during the early and late phase of formalin nociception: analysis using cerebral blood flow in the rat. Pain. 1998;75:355–365. doi: 10.1016/s0304-3959(98)00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary–adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol. 1995;16(2):89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Palecek J, Palecková V, Dougherty PM, Carlton SM, Willis WD. Responses of spinothalamic tract cells to mechanical and thermal stimulation of skin in rats with experimental peripheral neuropathy. J Neurophysiol. 1992;67:1562–1573. doi: 10.1152/jn.1992.67.6.1562. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Morrow TJ, Casey KL. Bilateral behavioral and regional cerebral blood flow changes during painful peripheral mononeuropathy in the rat. Pain. 2000;84:233–245. doi: 10.1016/s0304-3959(99)00216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academic Press; 1986. [Google Scholar]
- Rasmusson DD, Louw DF, Northgrave SA. The immediate effects of peripheral denervation on inhibitory mechanisms in the somatosensory thalamus. Somatosens Mot Res. 1993;10:69–80. doi: 10.3109/08990229309028825. [DOI] [PubMed] [Google Scholar]
- Rho JH, Swanson LW. Neuroendocrine CRF motoneurons: intrahypothalamic axon terminals shown with a new retrograde-Lucifer-immuno method. Brain Research. 1987;436(1):143–147. doi: 10.1016/0006-8993(87)91566-6. [DOI] [PubMed] [Google Scholar]
- Riddoch G. The clinical features of pain. Lancet. 1938;234:1093–1098. (see also p. 1150–6 and 1205–9) [Google Scholar]
- Ro LS, Jacobs JM. The role of the saphenous nerve in experimental sciatic nerve mononeuropathy produced by loose ligatures: A behavioral study. Pain. 1993;52:359–369. doi: 10.1016/0304-3959(93)90170-T. [DOI] [PubMed] [Google Scholar]
- Ro LS, Chen ST, Tang LM, Jacobs JM. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79(2–3):265–274. doi: 10.1016/s0304-3959(98)00164-x. [DOI] [PubMed] [Google Scholar]
- Salimi I, Webster HH, Dykes RW. Neuronal activity in normal and deafferented forelimb somatosensory cortex of the awake cat. Brain Res. 1994;656(2):263–273. doi: 10.1016/0006-8993(94)91469-9. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Gruol D, Aldenhoff J, Pittman Q. Electrophysiological actions of corticotropin-releasing factor in the central nervous system. Fed Proc. 1985;44:237–242. [PubMed] [Google Scholar]
- Sotgiu ML, Biella G, Riva L. Poststimulus afterdischarges of spinal WDR and NS units in rats with chronic nerve constriction. NeuroReport. 1995;6:1021–1024. doi: 10.1097/00001756-199505090-00018. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Dextrorphan relieves neuropathic heat-evoked hyperalgesia in the rat. Neurosci Lett. 1993;151:107–110. doi: 10.1016/0304-3940(93)90058-s. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Neuropathic pain sensations are differentially sensitive to dextrorphan. NeuroReport. 1994;5:1438–1440. doi: 10.1097/00001756-199407000-00008. [DOI] [PubMed] [Google Scholar]
- Tal M, Eliav E. Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 1996;64:511–518. doi: 10.1016/0304-3959(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Treede R, Kenshalo DR, Gracely RH, Jones AKP. The cortical reprensentation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang J, Peterson M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995;73:1811–1820. doi: 10.1152/jn.1995.73.5.1811. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Studies on the spinal interaction of morphine and the NMDA antagonist MK-801 on the hyperesthesia observed in a rat model of sciatic mononeuropathy. Neurosci Lett. 1992;135:67–70. doi: 10.1016/0304-3940(92)90137-v. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


