Abstract
Purpose
To study associations between surgical outcome and mean postmenstrual age (PMA) when fibrovascular organization is detected between vascular and avascular retina following laser for acute retinopathy of prematurity (ROP).
Methods
PMA at the time of detection of fibrovascular organization was determined in infants who had laser treatment for stage 3 ROP. Retinal features abstracted from examination sheets included zone, stage, and clock hours of fibrovascular organization, PMA at the times of first surgery and diagnosis of fibrovascular organization, and outcomes (retinal attachment after one surgery and retinal attachment at follow-up). Statistical analyses were performed to compare categorical data (Mann-Whitney U test, t–test, Fisher exact test) and determine correlations (Spearman rank test).
Results
Fibrovascular organization was diagnosed in 38/39 eyes that required surgery and 19/41 eyes that did not. In surgical eyes, older PMA at the time of detection of fibrovascular organization, zone II ROP, and stage 4 (versus stage 5) ROP were each associated with successful reattachment of the retina after one surgery and at the end of follow-up. PMA at diagnosis of fibrovascular organization was associated with zone, but not stage, of ROP when surgical intervention was performed.
Conclusion
Fibrovascular organization between the vascular and avascular retina is important because it is associated with the development of retinal detachment after laser for acute ROP. Further study is required to determine if improved detection of fibrovascular organization in eyes of infants of early PMA will improve surgical outcomes for retinal detachment.
Keywords: prematurity, retinal surgery, retinopathy of prematurity, ROP, fibrovascular organization, stage 4, stage 5, zone, postmenstrual age
Untreated retinal detachment after laser treatment for acute retinopathy of prematurity (ROP) results in poor visual outcomes in premature infants. Predicting which eyes are at the greatest risk for retinal detachment would therefore allow early intervention and potentially improved visual outcomes. Several groups have studied ROP after laser treatment, including the timing of involution1 and retinal features predictive of progressive stage 4 ROP.2 These studies emphasized the importance of monitoring the tissue between the vascular and avascular retina, which has been clinically defined as a thickened ridge,2,3 adjacent vitreous,1 or fibrovascular organization.1–3 Vitreous state (the presence of haze, hemorrhage, or vitreous organization over the optic nerve) and plus disease predicted both progressive stage 4 ROP and outcome after surgical intervention for retinal detachment. However, the only feature that predicted progressive retinal detachment requiring surgical intervention was clock hour extent of the thickened ridge,2 which is referred to in this study as fibrovascular organization.
Because fibrovascular organization was already shown to be a specific and important predictive feature, we were interested in the postmenstrual age (PMA) at diagnosis of fibrovascular organization following laser treatment for ROP to determine possible associations with outcome after surgery for retinal detachment. Fibrovascular organization can be better detected on clinical examination than with wide angle digital imaging. Current technology lacks the resolution to detect subtle fibrovascular organization and higher resolution imaging often requires infants to be imaged under general anesthesia to assure good focus. Fibrovascular organization can be quantified by clock hour extent and may be a practical feature to study in a multicenter clinical trial to determine the features predictive of progressive ROP.
Patients and Methods
The sample included 41 infants who had had fibrovascular organization diagnosed after laser for stage 3 ROP. Infants were referred for management of stages 3 to 5 ROP over the years from 1993 through 2004.2,3 All infant eyes had had carefully recorded retinal drawings before and after laser treatment for threshold or Type 1 prethreshold ROP4,5 by credentialed examiners for the CRYO-ROP study.6 The retinal drawings, based on forms from the International Classification of Retinopathy of Prematurity,7 had been developed to record retinal features before and after treatment for Type 1 prethreshold4,5 or threshold ROP.6,8 The ages when fibrovascular organization was detected and when surgery for retinal detachment was performed in those eyes that showed progressive ROP were recorded as PMA in weeks (gestational age plus chronologic age). Indications for surgery included vascularly inactive eyes with progressive stage 4 ROP threatening the macula, dense vitreous hemorrhage, or stage 5 ROP, and these indications were consistent throughout the study period. Surgical procedures included lens-sparing vitrectomy, scleral buckle (with later segmentation or removal), pars plicata vitrectomy, and open sky vitrectomy. All infants were followed for 4 months after laser to detect late progression to retinal detachment. We defined retinal attachment after the first surgery as successful surgical reattachment of the retina 4 months after one surgery. If the retina was detached 1 month after the first surgery, additional surgery was considered. Retinal attachment at the end of follow-up was retinal attachment at 4 months after all surgeries, including eyes that had fibrovascular organization, regression, and did not require surgery for retinal detachment.
Fibrovascular organization was defined as elevated and thickened tissue at the junction of avascular and vascular retina that had components of fibrovascular proliferation and vitreous organization. We refer to this feature throughout this study as fibrovascular organization. Fibrovascular organization was diagnosed at least 2 weeks following laser for acute ROP based on a study in which retinal detachment did not develop before 2 weeks following laser.1 Eyes included those that had not shown regression following laser as well as eyes that had apparent recurrent thickening after treatment. Sometimes the diagnosis of fibrovascular organization was made when neovascularization had regressed sufficiently to detect fibrovascular organization at the junction. Fibrovascular organization was counted in clock hour extent and was recorded separate from clock hours of neovascularization or retinal detachment (Figure 1). Because some infants were referred for management of stage 4 ROP after treatment for laser, the time of diagnosis of fibrovascular organization may have been later than the actual occurrence, but was within a range of 2 weeks based on review and comparison of the dates when laser and surgery were performed for each eye.
Fig. 1.
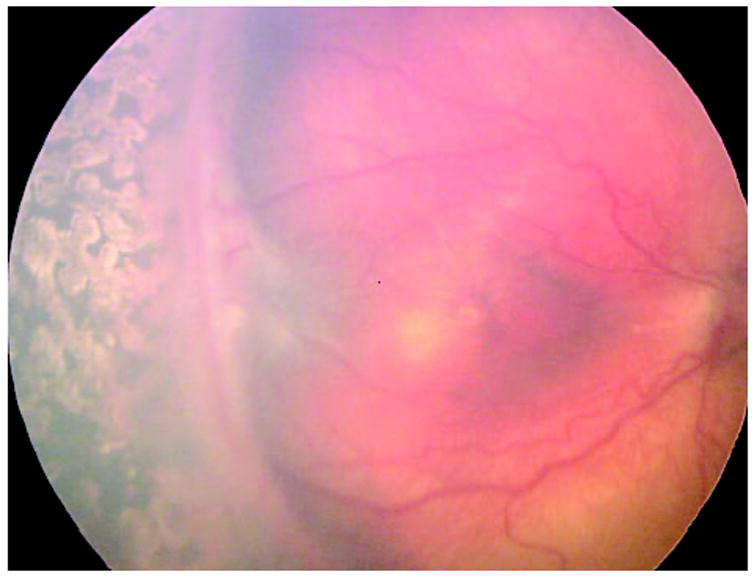
Wide angle image (Retcam) of right eye of premature infant demonstrating obvious fibrovascular organization from approximately 7 to 10:30 o’clock. There is 1 clock hour of retinal detachment posterior to fibrovascular organization from 8:30 to 9:30. (Retcam image provided by Sharon Freedman, MD, Duke University, Durham, NC.)
A database was created based on demographic data of patients (gestational age, birthweight, sex), retinal features abstracted from examination sheets including zone, stage, and clock hours of fibrovascular organization, PMA at the time of first surgery and at the time of diagnosis of fibrovascular organization, and outcomes.
Human subjects’ approval for exempt status was obtained to retrospectively gather data from patient charts. All patients received full examinations, including assessment of adequacy of laser and the presence of skip lesions.
Statistical Analysis
Summary data are presented as means and standard deviations (SDs) or median and interquartile range (IQR) as appropriate. Statistical analysis was performed using t-tests or Mann-Whitney U tests. Fisher exact tests were used to compare categorical data, and correlations were tested using the Spearman rank test. There was no adjustment for eyes in the same infants because the study was performed to derive estimates of times of diagnosis of fibrovascular organization in association with surgical outcome. However, the differences between mean PMA at diagnosis of fibrovascular organization in right versus left eyes, including the bilateral and unilateral cases, were analyzed to determine possible bias in the sample, and none was found.
Results
This study included 80 eyes (39 pairs) from 41 infants (15 female, 26 male). The median birth-weight was 681 g (interquartile range [IQR]: 614 –761 g) and gestational age 24 weeks (IQR: 24 –25 weeks). In all but one eye that developed vitreous hemorrhage before threshold ROP, previous laser treatment for threshold6,8 or Type 1 prethreshold4,5 ROP was performed as recommended. All infants had been treated with laser, and no apparent skip lesions were noted in any eye. All eyes, except one, which developed stage 4A ROP, did so 2 or more weeks after laser. In the one exception, the infant developed a stage 4B tractional retinal detachment within 1 week of laser.
Thirty-nine eyes developed progressive retinal detachment and required surgery, whereas 41 eyes showed regression of ROP. Because of the referral nature of the practice, the frequency of retinal detachment is much higher than that reported in studies that report the frequency of progression of ROP after laser.6,8 For example, some patients who received laser treatment by outside ophthalmologists were never referred because stage 3 ROP regressed without the development of retinal detachment, whereas other infants were specifically referred for management of retinal detachment. Thirteen infants had bilateral surgery, 12 unilateral surgery, and 14 had no surgery. An additional 2 infants had information only on one eye; one infant had surgery and the other did not. The PMA at the time of the first surgery ranged from 35 to 105 weeks (Table 1).
Table 1.
Characteristics of Eyes Undergoing Surgery for Retinal Detachment After Laser Treatment for ROP
| ID | Sex | GA, wk | Birthweight, g | Procedures | Stage | Zone | RA After First Surgery | PMA First Surgery, wk | RA at Follow-Up | PMA Diagnosis of Fibrovascular Organization | Clock Hours of Fibrovascular Organization |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 24.0 | 624 | OSV | 5 | 1 | N | 44 | N | 36 | 3 |
| 2 | F | 24.0 | 624 | OSV | 5 | 1 | N | 58 | N | 36 | 3 |
| 3 | F | 26.5 | 770 | SB, OSV, SPLIT SB | 4b | 2 | N | 37 | N | 37.5 | 5 |
| 4 | M | 23.0 | 653 | SB, OSV | 4a | 1 | N | 37 | N | 35 | 6 |
| 5 | M | 24.0 | 660 | LSV, OSV | 4a | 1 | N | 37 | N | 36 | 6 |
| 6 | M | 24.0 | 660 | OSV, PPV, PPV | 5 | 1 | N | 54 | N | 36 | 6 |
| 7 | M | 24.0 | 700 | SB, OSV, PPV SB, SPLIT SB, OSV, | 4b | 1 | N | 37 | N | 36 | 8 |
| 8 | M | 24.5 | 633 | PPV | 4b | 2 | N | 42 | N | 38 | 9 |
| 9 | M | 24.0 | 700 | OSV | 5 | 1 | N | 58 | N | 37 | 12 |
| 10 | M | 23.0 | 653 | OSV | 5 | 1 | N | 105 | N | 37 | 12 |
| 11 | M | 25.5 | 730 | PPV, SB, SPLIT SB SB, OSV, REMOVE | 5 | 1 | N | 38 | N | 37.5 | 12 |
| 12 | M | 23.5 | 480 | SB | 4a | 2 | N | 38 | N | 39 | 12 |
| 13 | F | 24.5 | 2380 | SB, OSV | 5 | 2 | N | 42 | N | 43 | 12 |
| 14 | M | 25.0 | 766 | LSV, SB, PPV, PPV | 4b | 1 | N | 51 | N | 51 | 12 |
| 15 | M | 24.0 | 737 | PPV—lensectomy | 5 | 1 | N | 44 | N | 44 | Unknown |
| 16 | F | 24.0 | 568 | SB, SPLIT SB, LSV | 4b | 1 | N | 38 | Y | 37 | 8 |
| 17 | M | 24.0 | 730 | LSV, OSV SB, SPLIT SB, OSV, | 4b | 1 | N | 37 | Y | 36 | 12 |
| 18 | M | 24.0 | 730 | PPV, PPV OSV, PPV,SB,PPV,SPLIT | 4b | 1 | N | 38 | Y | 36 | 12 |
| 19 | M | 25.5 | 730 | SB, REMOVE SB SB, SPLIT SB, OSV, | 5 | 1 | N | 51 | Y | 37.5 | 12 |
| 20 | M | 23.5 | 480 | SB, SPLIT SB | 4b | 2 | N | 37 | Y | 39 | 12 |
| 21 | F | 23.5 | 720 | NS | NS | 2 | NS | NS | Y | N | 0 |
| 22 | F | 26.0 | 564 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 23 | F | 27.0 | 454 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 24 | F | 27.0 | 454 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 25 | F | 27.0 | 740 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 26 | M | 24.0 | 590 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 27 | M | 24.0 | 590 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 28 | F | 26.5 | 770 | NS | NS | 2 | NS | NS | Y | N | 0 |
| 29 | F | 24.0 | 630 | NS | NS | 2 | NS | NS | Y | N | 0 |
| 30 | M | 25.0 | 950 | NS | NS | 2 | NS | NS | Y | N | 0 |
| 31 | F | 24.0 | 593 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 32 | M | 24.0 | 620 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 33 | M | 24.0 | 620 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 34 | F | 24.0 | 614 | NS | 3 | 1 | NS | NS | Y | N | 0 |
| 35 | F | 24.0 | 614 | NS | 3 | 1 | NS | NS | Y | N | 0 |
| 36 | F | 24.0 | 610 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 37 | F | 24.0 | 610 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 38 | M | 24.0 | 681 | NS | NS | 2 | NS | NS | Y | N | 0 |
| 39 | F | 26.0 | 920 | NS | NS | 2 | NS | NS | Y | N | 0 |
| 40 | M | 25.0 | 639 | NS | NS | 2 | NS | NS | Y | N | 0 |
| 41 | M | 28.0 | 1184 | NS | 3 | 2 | NS | NS | Y | N | 0 |
| 42 | F | 24.3 | 605 | NS | 3 | 2 | NS | NS | Y | 36 | 1 |
| 43 | M | 23.5 | 599 | NS | 3 | 1 | NS | NS | Y | 37 | 1 |
| 44 | M | 26.0 | 920 | NS | 3 | 2 | NS | NS | Y | 41 | 1 |
| 45 | F | 24.5 | 2380 | NS | 4a | 2 | NS | NS | Y | 43 | 1 |
| 46 | F | 24.3 | 605 | NS | 3 | 2 | NS | NS | Y | 36 | 2 |
| 47 | M | 23.5 | 599 | NS | 3 | 1 | NS | NS | Y | 37 | 2 |
| 48 | F | 24.0 | 593 | NS | 3 | 2 | NS | NS | Y | 42 | 2 |
| 49 | F | 27.0 | 740 | NS | 3 | 2 | NS | NS | Y | 43 | 2 |
| 50 | M | 24.3 | 700 | NS | 3 | 2 | NS | NS | Y | 43 | 2 |
| 51 | M | 24.3 | 700 | NS | 3 | 2 | NS | NS | Y | 43 | 2 |
| 52 | F | 27.0 | 761 | NS | 4a | 2 | NS | NS | Y | 42 | 3 |
| 53 | M | 24.0 | 530 | NS | 3 | 2 | NS | NS | Y | 42 | 3 |
| 54 | M | 26.0 | 920 | NS | 3 | 2 | NS | NS | Y | 41 | 4 |
| 55 | M | 25.0 | 624 | NS | NS | 2 | NS | NS | Y | 41 | 4 |
| 56 | M | 25.0 | 950 | NS | NS | 2 | NS | NS | Y | 42 | 4 |
| 57 | F | 24.0 | 568 | NS | NS | 1 | NS | NS | Y | 39 | 5 |
| 58 | M | 24.0 | 680 | NS | 3 | 2 | NS | NS | Y | 35 | 6 |
| 59 | M | 25.0 | 624 | NS | NS | 2 | NS | NS | Y | 41 | 6 |
| 60 | M | 24.0 | 681 | NS | NS | 2 | NS | NS | Y | 44 | 10 |
| 61 | M | 24 | 740 | NS | NS | 1 | NS | NS | Y | N | N |
| 62 | F | 27.0 | 761 | LSV,PPV | 4a-b | 2 | Y | 42 | Y | 42 | 4 |
| 63 | F | 26.0 | 564 | LSV | 4a | 2 | Y | 48 | Y | 44 | 4 |
| 64 | M | 24.0 | 680 | LSV | 4a-4b | 2 | Y | 35 | Y | 35 | 5 |
| 65 | M | 24 | 740 | LSV, SB | 4a-4b with exudation | 1 | Y | 36 | Y | 35 | 5 |
| 66 | M | 28.0 | 1184 | LSV | 4a-b | 2 | Y | 50 | Y | Unknown | 5 |
| 67 | M | 24.0 | 908 | LSV | 4b | 2 | Y | 42 | Y | 40 | 5.5 |
| 68 | M | 24.0 | 530 | LSV | 4a | 2 | Y | 42 | Y | 42 | 6 |
| 69 | M | 24.0 | 908 | SB, SPLIT SB | 4b | 2 | Y | 41 | Y | 40 | 6.5 |
| 70 | M | 24.0 | 737 | LSV | 4b | 1 | Y | 44 | Y | 42 | 8 |
| 71 | M | 25.0 | 639 | SB, SPLIT SB, LSV | 4b | 2 | N | 39 | Y | 39 | 6 |
| 72 | M | 24.0 | 870 | SB, SPLIT SB, LSV | 4a | 2 | N | 40 | Y | 39 | 7 |
| 73 | M | 24.5 | 633 | SB, SPLIT SB, OSV | 5 | 2 | N | 38 | Y | 38 | 10 |
| 74 | M | 24.0 | 780 | SB, OSV, PPV, SPLIT SB | 4b | 2 | N | 40 | Y | 40 | 12 |
| 75 | F | 23.5 | 720 | SB, SPLIT SB | 4b | 2 | Y | 48 | Y | 44 | 2 |
| 76 | M | 24.0 | 695 | SB, SPLIT SB | 5 | 2 | Y | 40 | Y | 40 | 4 |
| 77 | F | 24.0 | 630 | SB, SPLIT SB | 4a | 2 | Y | 35 | Y | 36 | 8 |
| 78 | M | 24.0 | 870 | SB, SPLIT SB, LSV | 4b | 2 | Y | 40 | Y | 39 | 8 |
| 79 | M | 24.0 | 695 | SB, SPLIT SB | 4b | 2 | Y | 41 | Y | 40 | 8 |
| 80 | M | 24.0 | 780 | SB, REMOVE SB | 4b | 2 | Y | 41 | Y | 40 | 12 |
ROP = retinopathy of prematurity; ID = patient number; GA = gestational age; RA = retinal attachment; PMA = postmenstrual age in weeks; OSV = open-sky vitrectomy; SB = scleral buckle; SPLIT SB = segmentation of SB; LSV = lens-sparing vitrectomy; PPV = pars plicata vitrectomy.
Detection of fibrovascular organization was made in 57 infant eyes and was unknown in one. Of the 57 eyes, 6 or more clock hours were found in 30 eyes. Fibrovascular organization was diagnosed in 38 of 39 eyes that required surgery (information not known in one eye requiring surgery) compared to 19 eyes that did not have surgery. Of those eyes that required surgery, 69% (27/39) had >6 clock hours of fibrovascular organization, whereas in eyes that did not require surgery, only 3/41 eyes (7%) had 6 or more clock hours of fibrovascular organization (Table 1).
There was no difference between right and left eyes for mean PMA at time of diagnosis of fibrovascular organization or first surgery. The mean PMA ± SD at time of diagnosis of fibrovascular organization was 38.4 ± 3.5 weeks (35–51 weeks; n = 24) in those eyes without retinal reattachment after one surgery, and 39.9 ± 2.9 weeks (35– 44 weeks; n = 14) in eyes that had retinal attachment at 4 months after one surgery (P = NS). When the data were reviewed (Figure 2), there was one eye in which the diagnosis of fibrovascular organization was made at 51 weeks PMA. When this outlier was removed and the data reanalyzed, a higher mean PMA at the time of diagnosis of fibrovascular organization was found in eyes with successful surgical reattachment after the first surgery (39.9 ± 2.9 weeks; P = 0.017, t-test) than in eyes without surgical attachment (37.8 ± 2.2 weeks). Significance was also found for higher mean PMA at diagnosis of fibrovascular organization (39.7 ± 2.8 weeks; n = 42) for eyes with retinal attachment at follow-up (including nonsurgical eyes with fibrovascular organization after laser) than eyes that remained detached at 4-month follow up (37.7 ± 2.7 weeks; n = 14; P = 0.024, t-test; Figure 3). In the group requiring surgery, there was no significant association in mean PMA at the time of the first surgery and surgical outcome (n = 39), including after the outlier of 105 weeks was removed (P = NS).
Fig. 2.
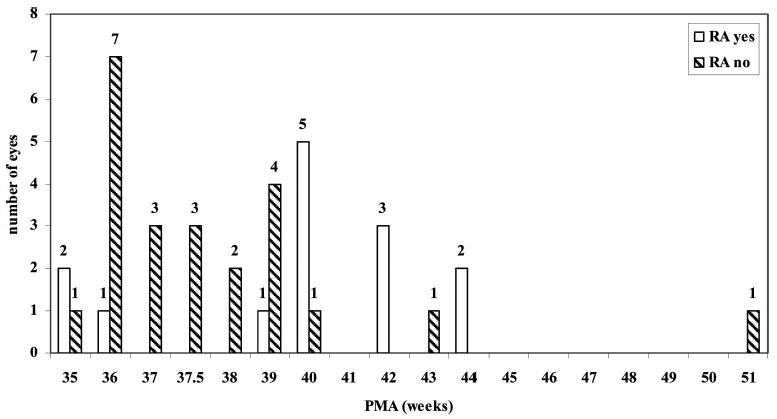
Postmenstrual age (PMA) at diagnosis of fibrovascular organization versus outcome after first surgery for retinal detachment. Infant eyes are separated into those having successful retinal attachment (RA) after one surgery or not.
Fig. 3.
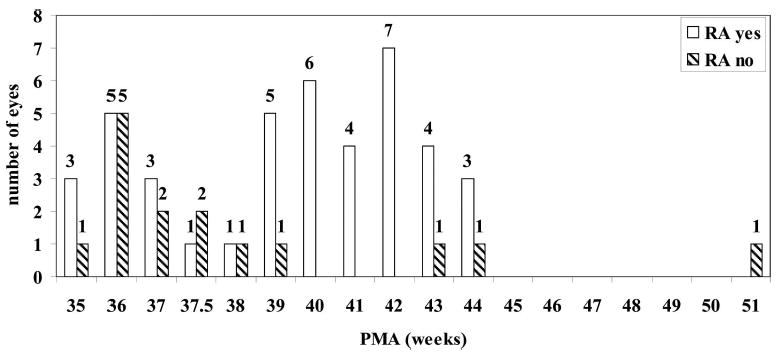
Postmenstrual age (PMA) at diagnosis of fibrovascular organization versus outcome at follow-up, including surgical and regressed, nonoperated eyes. Infant eyes are separated into those having successful retinal attachment (RA) after all surgeries or not.
When eyes were analyzed as to whether they had ≥6 clock hours of fibrovascular organization or <6 clock hours of fibrovascular organization, there was no difference in surgical outcomes after one or more surgeries.
When retinal attachment after the first surgery was analyzed by zone of ROP, there was a significant association between successful surgical attachment and zone II disease (P = 0.003, t-test). There was also a significant association between zone II ROP and retinal attachment after all surgeries at 4 months (P < 0.0001, t-test). There were positive associations of stage of ROP at first surgery and outcome after first surgery (RA first) (P = 0.028, Fisher exact test) and at the end of follow-up (RA any) (P = 0.008, Fisher exact test) with retinal attachment more likely in stage 4 than stage 5 eyes. However there was no correlation in PMA at diagnosis of fibrovascular organization and stage 4 or 5 ROP (Spearman rank correlation P = 0.536) or difference in median PMA at diagnosis of fibrovascular organization between stage 4 or 5 ROP (Figure 4A). PMA at diagnosis of fibrovascular organization was correlated with zone of ROP (Spearman rank correlation P < 0.001) with a higher PMA associated with zone II ROP (Figure 4B) (Mann-Whitney U, P < 0.0001).
Fig. 4.
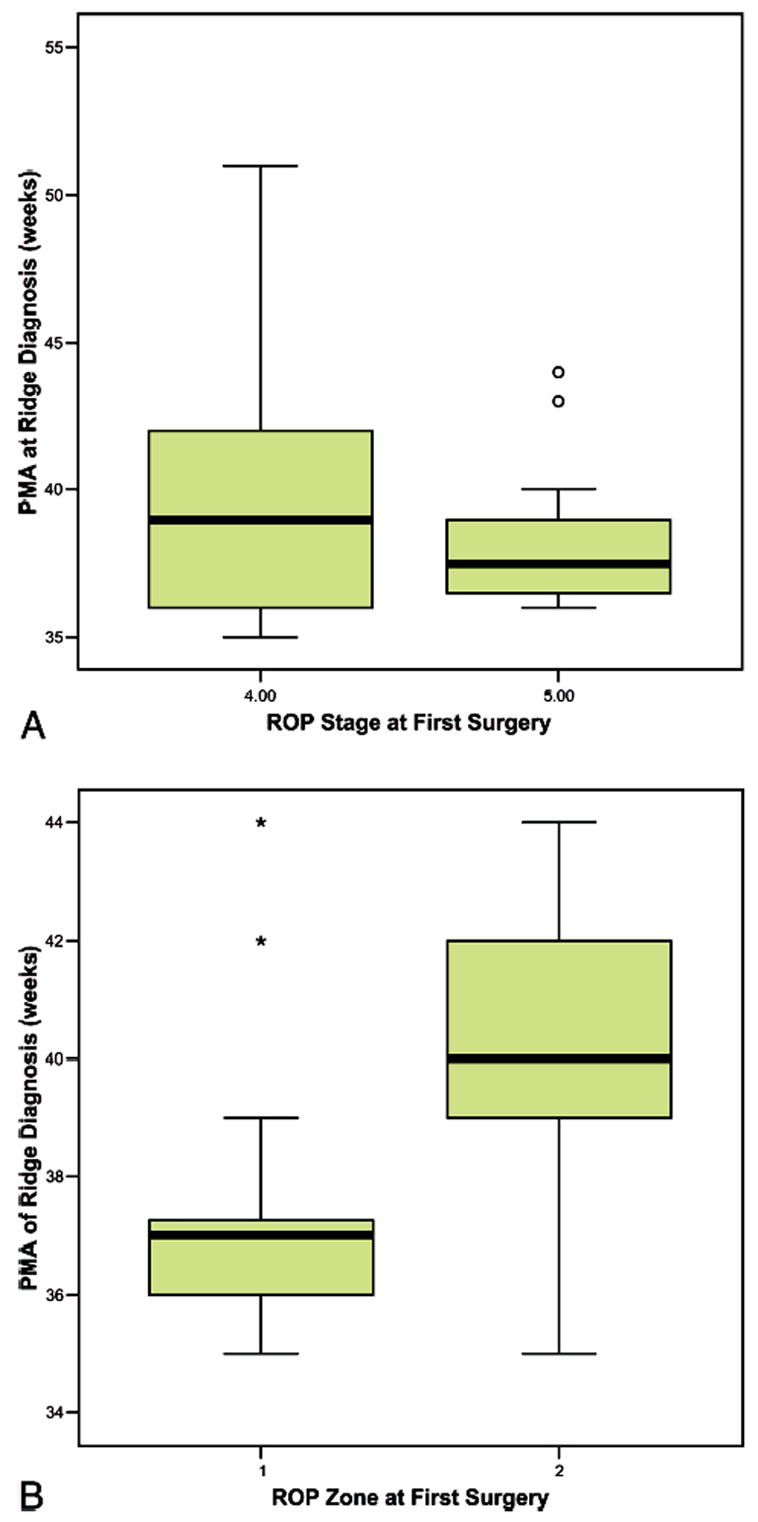
A, Box plot of postmenstrual age at diagnosis of fibrovascular organization versus retinopathy of prematurity (ROP) stage at first surgery (P = NS). B, Box plot of postmenstrual age at diagnosis of fibrovascular organization versus ROP zone at first surgery (P < 0.0001).
Discussion
The fibrovascular organization between vascular and avascular retina is an important feature in acute ROP. Fibrovascular organization has been shown to be an important feature in monitoring involution or predicting progression to retinal detachment.1,2 Detection of fibrovascular organization after adequate laser treatment predicts progressive retinal detachment by clock hour extent (≥6 clock hours)3 and, as we have shown in this article, outcome based on the PMA when detected. Fibrovascular organization is also quantifiable and detectable by examination or digital imaging, making it a useful feature to monitor treatment effects.
Our data support that the least mature infants and eyes have the poorest outcomes. In this study, we found that eyes in which detection of fibrovascular organization after laser occurred at a later PMA achieved successful retinal reattachment more often than did eyes in which diagnosis of fibrovascular organization occurred earlier. Furthermore, we found a strong correlation between zone I ROP and failed retinal reattachment following surgery. Zone and PMA at diagnosis of fibrovascular organization were also correlated. These data support that extremely premature infants or immature eyes, i.e., zone I with the least complete intraretinal vascularization, respond poorly,9 including to surgical intervention. On the other hand, although stage at first surgery did correspond to surgical outcome, it was not associated with the PMA at diagnosis of fibrovascular organization or maturity of the eyes or infants. The smallest eyes may have poor outcomes because they are technically more difficult to operate on because of their size and deformability. Persistent tunica vasculosa lentis can cause media opacity, and engorged iris vessels may impair the ability of the iris to dilate. Both these conditions impede visualization to the posterior segment. In addition, biologic, nontechnical factors may play a role in progression of retinal detachment and surgical outcome.10
We had too few eyes to determine whether delay between diagnosis of fibrovascular organization and timing of surgery affected surgical outcome. Delay of surgery may occur in infants because of other severe medical problems or because of the need to transport an infant to a center where pediatric vitreoretinal surgery is performed. In addition, unclear media from extensive tunica vasculosa lentis or vitreous hemorrhage may delay the diagnosis of retinal detachment. Vitreous hemorrhage has been reported associated with retinal detachment in 75% of cases of infants with treated ROP in one series.1 Delay may result in more progressive fibrovascular changes in the eye with acute ROP, including vascular activation or anterior extension of the fibrovascular tissue to the lens. Vascular activity is associated with poor surgical outcomes.3 Surgical intervention before the anterior extension of fibrovascular growth with traction causing the retina to be pulled towards the lens is technically easier and is more likely to permit the surgeon to spare the lens in vitrectomy.11–13
Fibrovascular organization between vascular and avascular retina is a unique feature in that it is relatively easy to quantify, unlike vitreous state, and was shown to be a predictor of progressive stage 4 ROP independent of surgical outcome.3 However it can be subtle to detect without scleral depression and visualization in cross-section. In this study, we found that older PMA at the time of fibrovascular organization was associated with better surgical outcomes supporting other studies that the most premature infants and immature eyes have the most guarded prognoses.9 However it is also possible that fibrovascular organization in eyes with zone I ROP from infants of young PMA is difficult to detect because scleral depression and visualization of fibrovascular organization in cross-section is difficult in posterior disease. Prospective study of fibrovascular organization and other features may be important to determine a window of surgical intervention in infants with stage 4 ROP or to monitor the effect of future strategies in preventing progressive retinal detachment. The PMA at detection of fibrovascular organization may also be helpful in choosing between surgery and future medical therapies10,14 as they are developed. Care must be taken in interpreting these results because they are from a small study and are retrospective.
Footnotes
Study performed at Schepens’ Retina Associates, Boston, Massachusetts; Department of Ophthalmology, Louisiana State University, New Orleans; and Department of Ophthalmology, University of North Carolina, Chapel Hill.
None of the authors has any financial or proprietary interests in any of the products or instruments used in this study.
Sponsoring organizations: Research to Prevent Blindness and NEI R01 EY015130-01A1.
References
- 1.Coats DK, Miller AM, Brady McCreery KM, et al. Involution of threshold retinopathy of prematurity after diode laser photocoagulation. Ophthalmology. 2004;111:1894–1898. doi: 10.1016/j.ophtha.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Hartnett ME, McColm JR. Retinal features predictive of progression to stage 4 ROP. Retina. 2004;24:237–241. doi: 10.1097/00006982-200404000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartnett ME. Features associated with surgical outcome in patients with stages 4 and 5 retinopathy of prematurity. Retina. 2003;23:322–329. doi: 10.1097/00006982-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Fielder AR. Preliminary results of treatment of eyes with high-risk prethreshold retinopathy of prematurity in the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1769–1771. doi: 10.1001/archopht.121.12.1769. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 6.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: Snellen visual acuity and structural outcome at 51/2 years after randomization. Arch Ophthalmol. 1996;114:417–424. doi: 10.1001/archopht.1996.01100130413008. [DOI] [PubMed] [Google Scholar]
- 7.The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 8.The STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial I: Primary outcomes . Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 9.Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology. 1993;100:230–237. doi: 10.1016/s0161-6420(93)31665-9. [DOI] [PubMed] [Google Scholar]
- 10.Trese MT, Capone A. Retinopathy of prematurity: evolution of stages 4 and 5 ROP and management. A. Evolution to retinal detachment and physiologically based management. In: Hartnett ME, Trese MT, Capone A, Keats BJK, Steidl SM, editors. Pediatric Retina. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 411–416. [Google Scholar]
- 11.Hartnett ME, Maguluri S, Thompson HW, et al. Comparison of retinal outcomes after scleral buckle or lens-sparing vitrectomy for stage 4 retinopathy of prematurity. Retina. 2004;24:753–757. doi: 10.1097/00006982-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Prenner JL, Capone AJ, Trese MT. Visual outcomes after lens-sparing vitrectomy for stage 4A retinopathy of prematurity. Ophthalmology. 2004;111:2271–2273. doi: 10.1016/j.ophtha.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard GB, III, Cherwick DH, Burian G. Lens-sparing vitrectomy for stage 4 retinopathy of prematurity. Ophthalmology. 2004;111:2274–2277. doi: 10.1016/j.ophtha.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]


