Abstract
Purpose
To study the effects of intravitreous triamcinolone acetonide (TA) on neovascularization (NV), capillary density, and retinal endothelial cell (REC) viability in a model of oxygen-induced retinopathy (OIR).
Methods
Newborn rats exposed to OIR underwent intravitreous injections (right eye) at day 14 to achieve intravitreous concentrations of: dexamethasone (DEX) (0.3 mg/mL), triamcinolone (TA; 0.4–4 mg/mL), or PBS. Animals were removed to room air and at day 18, retinal flatmounts were assayed for clock hours of NV, percent peripheral avascular retina, capillary density, apoptosis, and VEGF protein. At day 15, retinas were assayed for insulin-like growth factor (IGF)-1 receptor phosphorylation (IGF-1Rphos). Human RECs exposed to TA were assayed for trypan blue exclusion or activated caspase-3.
Results
TA but not DEX or PBS reduced NV (ANOVA, P < 0.001), capillary density (ANOVA, P < 0.001), and systemic weight gain (ANOVA, P = 0.002). VEGF protein was not different between TA- and PBS-injected or noninjected groups. Apoptosis was not increased in vivo or in vitro between groups, but there was a dose-dependent toxic effect of TA on cultured RECs (P < 0.001). At day 15, retinas from the 4 mg/mL TA-injected OIR group had a trend toward reduced IGF-1Rphos compared with room air–raised PBS- or non-injected OIR groups.
Conclusions
TA caused dose-dependent reductions in NV, retinal vascularization, and systemic weight gain associated with a reduction in IGF-1Rphos. Long-term studies are needed to assess TA toxicity in vivo. TA doses should be carefully considered before administering the drug in diseases with ongoing retinal vascular development, such as retinopathy of prematurity.
Increasing evidence links inflammation to retinovascular diseases. Several studies have shown that ocular fluid from diabetics have measurable inflammatory cytokines,1,2 and in an animal model of retinal neovascularization (NV), IL-6, and TNF-αwere temporally associated with subsequent release of angiogenic growth factors and the development of intravitreous NV.3 Inflammatory cells, such as leukocytes, have been implicated in the creation of avascular retinal areas in animal models of diabetes through endothelial cell apoptosis.4,5 These avascular retinal regions have been postulated to be the stimulus for NV6 through the overexpression of angiogenic growth factors,7 including vascular endothelial growth factor (VEGF). Conversely, an isoform of VEGF has been shown to increase leukocyte adherence to endothelial cells (ECs) leading to apoptosis and retinal avascularity.8 Thus, inflammation has been shown to cause NV through release of inflammatory cytokines and through the creation of avascularity, both linked to the increased expression of angiogenic factors.
Anti-inflammatory therapy has been used to reduce NV in clinical inflammatory diseases such as posterior uveitis.9 Corticosteroids have been shown to downregulate induced VEGF in cultured Müller cells and in an RPE cell line.10,11 In addition, in a mouse model of oxygen-induced vaso-obliteration,12 the corticosteroid triamcinolone (TA) reduced capillary proliferation into the vitreous. Based on these findings, local delivery of corticosteroids into the eye has been considered to treat several retinovascular diseases.13–15 Clinical trials are under way to test intravitreous TA (1 mg/mL in vitreous) for macular edema in diabetic retinopathy and retinal vein occlusion.13 In addition, some investigators have tested the effects of TA in persistent NV after laser treatment in retinopathy of prematurity (ROP; Tawansy KA, et al. IOVS 2005;46:ARVO E-Abstract 3493). Besides the known clinical risks of cataract formation and increased intraocular pressure, recent studies have shown a toxic effect of TA on several retinal cell lines.16 To understand further the effect of TA in vivo, we used a well-accepted animal model of oxygen-induced retinopathy (OIR)17 that uses oxygen extremes similar to that of a preterm infant at risk for severe ROP,18 to study the effect of intravitreous injection of TA on NV, capillary density, and EC viability.
In our short-term study, we found a dose-related decrease in NV and capillary density of the vascularized retina that appeared to be related to a delay in vascularization and the signaling pathway of insulin-like growth factor (IGF)-1. We also found a significant dose-dependent decrease in systemic weight gain of pups given intravitreous TA. Our findings may be relevant to the use of intravitreous TA in the management of retinovascular diseases, particularly those with ongoing intraretinal vascular development, such as ROP.
Methods
All animals were cared for in accordance with the University of North Carolina’s Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Drug Preparations
The doses of dexamethasone (DEX; American Pharmaceutical Partners, Inc., Los Angeles, CA) and TA acetonide (TA; Bristol Myers Squibb, New York, NY) were chosen based on comparable doses used clinically in human eyes for macular edema19 or inflammation.20 The vitreous concentrations reported for adult human diseases are 0.1 mg/mL DEX and between 1.0 and 6.25 mg/mL TA.12,19,20 Based on an estimated vitreous volume of 15 μL in a postnatal day (P)14 rat,21 the effective vitreous concentrations used in this study were: DEX, 0.3 mg/mL; and TA, 0.4, 1, 2, and 4 mg/mL. A greater concentration of DEX than is recommended in human disease was chosen, to be commensurate with glucocorticoid inhibitory effects of the doses of TA most often used, given that DEX is approximately five times more potent than TA. The concentration of the preservative, benzalkonium chloride, used in commercially available TA has been reported as nontoxic to ocular tissue in some studies (Kube T, et al. IOVS 2005; 46:ARVO E-Abstract 485), whereas in another study, only three times the concentration of the preservative, benzyl alcohol, was shown to be toxic in rabbit retinas.22 Therefore, the suspensions of TA doses were purified by centrifugation for 5 minutes, to reduce the potential toxicities of the benzyl alcohol and benzalkonium chloride vehicles. The supernatant was discarded, and the drug was reconstituted at the desired concentration in sterile 1 M phosphate-buffered saline (PBS). Centrifugation was chosen as the desired purification technique, because it has been reported to remove the solvent agent effectively without significantly lowering the dose of TA.23
In Vitro Studies of TA on Retinal Microvascular EC Survival
Doses chosen for in vitro experiments were based on those used in previous studies.11,12,16,24 Survival was assessed by either lack of staining with activated caspase-3 or exclusion of trypan blue after exposure to TA for 24 hours. Primary human retinal microvascular ECs (RECs; Cell Systems, Kirkland, WA) were plated (3 × 104 cells/cm2) in DMEM-low glucose containing 10% fetal bovine serum (FBS), EC growth supplement (120 μg/mL, Sigma-Aldrich, St. Louis, MO), and heparin (100 μg/mL; Sigma-Aldrich) and allowed to adhere overnight on pre-coated coverslips (Attachment Factor; Cell Systems). After attachment overnight, the media were changed to 5% FBS with the growth factors and 0, 20, 200, or 2000 μg/mL TA and incubated for 24 hours. The positive control for apoptosis was staurosporine (1 μg/mL, Sigma-Aldrich) added 6 hours before assay. The cells were fixed in 2% paraformaldehyde (PFA; Sigma-Aldrich), rinsed, and incubated in PBS+0.5% Triton X-100 (Sigma-Aldrich) for 30 minutes. After the reaction was preblocked with PBS+3% BSA (Sigma-Aldrich) for 30 minutes at room temperature, the coverslips were incubated overnight in FITC-conjugated cleaved caspase-3 antibody (Cell Signaling Technology, Beverly, MA) diluted 1:100 in PBS+3% BSA at 4°C. The samples were washed three times in PBS for 10 minutes and incubated in Hoechst 33342 (1:5000; Invitrogen-Molecular Probes, Eugene, OR) for 30 minutes. After three more washes in PBS, the coverslips were mounted on slides in PBS+glycerol (2:1, Vectashield; Vector Laboratories, Burlingame, CA) and viewed with a scanning confocal microscope at the Michael-Hooker Microscopy Facility, University of North Carolina at Chapel Hill; SP2; Leica, Deerfield, IL).
To measure cell death, treated RECs were incubated in 0.25% trypsin (Invitrogen, Carlsbad, CA) for 5 minutes at 37°C. After quenching by adding DMEM with 10% FBS, 0.4% trypan blue solution (1:5, ICN Biomedicals, Aurora, OH) was added, and the cells were counted with a hemocytometer. Cell viability was calculated as the number of live cells (those that excluded trypan blue) expressed as a percentage of total cells.
Model of OIR
A bioactive gas controller (Oxycycler; BioSpherix, New York, NY), which regulates the atmosphere inside an incubator by injecting either nitrogen or oxygen, was used to induce OIR in newborn Sprague-Dawley rats (Charles River, Wilmington, MA) as reported.17 Within a few hours of birth, pups designated postnatal age (P)0 and their mothers were placed into the incubator. Oxygen was cycled between 50% and 10% every 24 hours for 14 days, and then pups were returned to room air for four additional days (50/10 OIR model).17 These oxygen extremes were similar to transcutaneous oxygen measurements in a premature infant with severe ROP.18 As the rat blood oxygen level (Pa O2) is directly correlated with inspired oxygen, this model is an excellent mimic of the oxygen insult that premature infants experience.25 Litters of 12 to 13 pups were used in all experiments. Carbon dioxide in the cage was monitored and flushed from the system by maintaining sufficient gas flow.
Intravitreous Injection of Corticosteroids
On removal from the gas controller (Oxycycler; BioSpherix), P14 rat pups were anesthetized with an intraperitoneal (IP) injection of a mixture of ketamine (20 mg/kg) and xylazine (6 mg/kg). Fused eyelids were carefully opened with forceps, and 0.5% tetracaine hydrochloride was administered to the exposed eye. With the eye gently proptosed, a 30-gauge needle was inserted just behind the limbus to avoid damaging the lens. One microliter of PBS, DEX, or TA was injected into the vitreous humor of one eye. The fellow eyes were noninjected and used as a control group, similar to published13 and current clinical trials. Delivery of TA was visually confirmed through the microscope by the presence of drug crystals in the vitreous. Topical antibiotic ointment (0.5% erythromycin) was then applied to the eye. After 4 days in room air, when NV was at its maximum,25 rats were killed, and the eyes were enucleated for subsequent analyses.
Dissecting Retinal Tissue for Flatmounting
Pups were anesthetized by IP injection of ketamine (20 mg/kg) and xylazine (6 mg/kg). PFA (0.7–1.0 mL, 0.5%) was directly perfused into the left ventricle before euthanatization by intracardiac injection of pentobarbital (80 mg/kg). Both eyes were enucleated and fixed in 2% PFA for 2 hours. In a modification of the method of Chan-Ling,26 the anterior segments were removed, and the retinas with the ora serrata intact were carefully dissected and placed into PBS, with care to remove the hyaloid vessels and any remaining vitreous. Each retina was then placed on a microscope slide and flattened by making four incisions, each 90° apart, beginning at the ora serrata and extending centrally from the equator, stopping short of the optic nerve opening.
Tissue Staining
The flattened retinas were permeabilized in ice-cold 70% (vol/vol) ethanol for 20 minutes and then in PBS/1% Triton X-100 for 30 minutes. The retinas were incubated with Alexa Fluor 586–conjugated Griffonia simplicifolia (Bandeiraea) isolectin B4 (5 μg/mL; Invitrogen-Molecular Probes) in PBS overnight at 4°C for staining of the vasculature. Some retinas were then incubated in cleaved caspase-3 (Asp175) antibody (5 μg/mL; Cell Signaling Technology) overnight at 4°C. The retinas were rinsed three times in PBS and mounted in PBS-glycerol (2:1, VectaShield; Vector Laboratories), and the coverslip was sealed with nail polish. Images of the superficial blood vessel layer were captured with an inverted microscope (TE2000U, Nikon, Tokyo Japan; Michael-Hooker Microscopy Facility, University of North Carolina, Chapel Hill) and digitally stored for analysis. Image sections were stitched with commercial image-management software (PhotoFit Premium ver. 1.44; Tekmate, Tokyo, Japan).
Counting Clock Hours of NV
Retinal images were randomized and divided into 12 clock hours of approximately equal area by using image-analysis software (Photoshop; Adobe Systems, Mountain View, CA). Each clock hour was then assessed by two masked examiners for the presence of NV, using an assessment technique adapted from those used in clinical trials27 and animal model determination,28 to assess vessel tufts. Each retina received a score of 0 to 12, based on the number of clock hours exhibiting any NV. This assessment was made on the superficial layer only and correlates well with counting preretinal tufts appearing above the inner limiting membrane of the rat retina.
Analysis of Peripheral Avascular Areas
Digitized images of the total retinal area and peripheral avascular areas were measured using the freeware ImageTool ver. 3 (University of Texas, Austin, TX). The peripheral avascular area was expressed as a percentage of the total retinal area.
Quantification of Capillary Density
Central capillary density was quantified by summing capillary junctions within four equal areas, each 0.16 mm2, in each of the four quadrants of the vascularized retina and was expressed as the number of junctions per 0.64 mm2, determined by ImageTool.
Fresh Tissue Preparation
Animals were euthanatized with an overdose of pentobarbital (80 mg/kg IP). Both eyes were enucleated, and the retinas were isolated under a dissecting microscope in a similar fashion as used for flat-mounting, except that the ora serrata was carefully removed. The tissue was placed in RIPA buffer (20 mM Tris base, 120 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol) for Western blot or M-Per for ELISA and frozen at −20°C until analysis.
Protein Extraction, Immunoprecipitation, and Western Blot Analysis
Freshly dissected unfixed retinal tissue immersed in RIPA buffer was placed on ice for 10 minutes. Tissue was homogenized, and lysates were centrifuged at maximum speed for 10 minutes at 4°C. For IGF-1R phosphorylation, the supernatant fractions from homogenized retinas were incubated with protein A Sepharose beads for preclearing at 4°C for 10 minutes, then removed by centrifugation. The Bradford assay (Bio-Rad, Hercules, CA) was performed to determine the protein concentration of the cell lysate. Cell lysates were incubated with IGF-1R antibody (cat no. sc-713; Santa Cruz Biotechnology Inc., Santa Cruz, CA) overnight. The immune complexes were captured by protein A Sepharose beads (GE Healthcare, Uppsala, Sweden) at 4°C for 2 hours, and the collected beads were washed three times by centrifugation. The bound proteins were eluted with sample buffer. Phosphorylated IGF-1R was detected by Western blot analysis, with anti-phosphotyrosine (cat. no. 9411; Cell Signaling Technology) and a goat anti-mouse HRP conjugated secondary (cat. no. AP130P; Chemicon, Temecula, CA). Visualization was performed with enhanced chemiluminescence (cat. no. 32109; Pierce, Rockford, IL). The signal intensity was quantitated with analysis software (UN-SCAN-IT, ver.6.1; Silk Scientific, Orem, UT).
ELISA
Tissue samples were thawed and subjected to centrifugation at 16,000g for 10 minutes at 4°C. The M-Per supernatant was removed and lysis buffer (0.15 M NaCl, 0.02 M Tris-HCl [pH 8.0], 1% NP40, 1% sodium deoxycholate, 0.1% SDS, 0.01% proteinase inhibitor cocktail [Sigma-Aldrich]) added to the sample. Samples were homogenized and centrifuged at 16,000g for 10 minutes at 4°C.
Total protein was quantified with a protein assay kit (Bio-Rad, Hercules, CA), which is a modification of the Lowry assay.29 Supernatants were then assayed without dilution, in duplicate, with a commercially available ELISA kit for VEGF (R&D Systems, Minneapolis, MN).
Statistical Analysis and Sample Sizes
Samples were compared using ANOVA with Bonferroni post hoc analysis (SPSS ver.14; SPSS, Chicago, IL). Three litters were used in each experimental group and within each litter, at least three animals were injected with each dose of corticosteroid or vehicle.
Results
Steroid Effect on Clock Hours of NV
DEX (intravitreous concentration of 0.3 mg/mL) compared to PBS-injected or noninjected eyes had no effect on the clock hours of NV (mean clock hours ± SD: 7.3 ± 2 vs. 8.6 ± 2.4 vs. 8.6 ± 2.0, respectively). There was no difference in clock hours of NV between TA (TA)-injected eyes, with or without vehicle at the 0.4 mg/mL intravitreous concentration (data not shown). Therefore, there did not appear to be an effect of vehicle on NV. However, based on other reports16,22 (Kube T, et al. IOVS 2005;46:ARVO E-Abstract 485), we performed all subsequent experiments using washed preparations as described in the Methods section.
TA caused a dose-dependent reduction in clock hours of NV compared with PBS-injected or noninjected eyes (overall ANOVA P < 0.001; Fig. 1). Since DEX did not reduce clock hours of NV at a dose higher than that used clinically, we performed further experimentation using only doses of TA.
Figure 1.
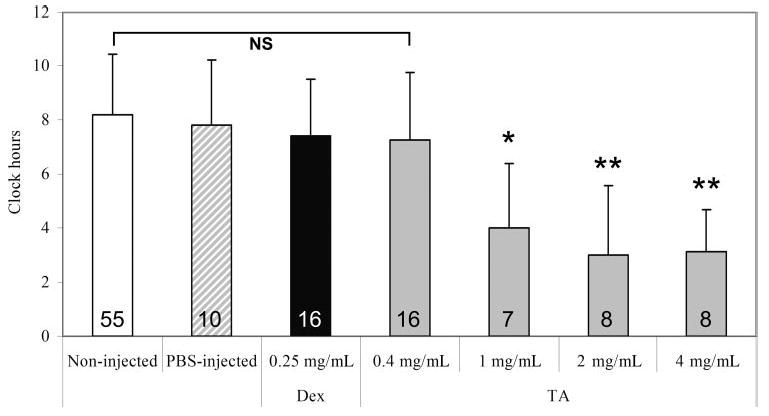
Effect of DEX or increasing doses of TA on clock hours of NV in rat eyes of a 50/10 OIR model (x-axis: final intravitreous concentrations). As noninjected eyes from all groups were not significantly different from each other, these data were pooled into one group termed “non-injected.” Overall ANOVA significance was set at P < 0.001. *P < 0.017, **P < 0.001 compared with noninjected, PBS, DEX and 0.4 mg/mL TA-injected. Numbers inside each bar represent the number of retinas analyzed per group.
Because there was no difference between the noninjected eyes from any of the TA doses, we pooled the data into one group labeled noninjected.
Steroid Effect on Peripheral Avascular Retinal Area and Capillary Density
There was no difference in measured peripheral avascular/total retinal area between noninjected or PBS-injected eyes and either DEX-injected or TA-injected eyes (data not shown). However, in the TA-injected eyes, there was a dose-dependent reduction in capillary density within the vascularized region of retina at P18 (ANOVA, P < 0.001; Figs. 2, 3). There was no difference in capillary densities in retinas of noninjected eyes at P14 compared with the those from TA-injected eyes at P18, suggesting a delay in further vascularization within the vascularized retina that naturally occurs in the 50/10 OIR model on rat pup return to room air (Fig. 2).
Figure 2.
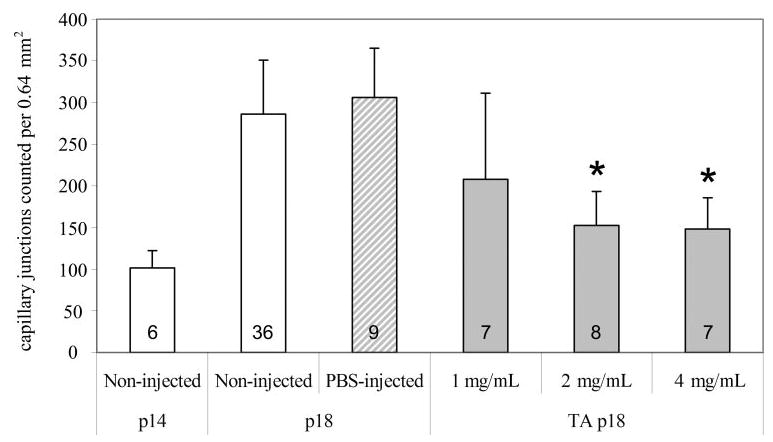
The effect of TA doses on capillary density (final intravitreous concentrations of TA: 1, 2, or 4 mg). All animals were cycled for the 50/10 OIR model. Some were killed at P14 without any intravitreous injections, and capillary density counts were made. Others were injected with TA at different doses, or vehicle (PBS), or were noninjected and returned to room air for 4 days before they were killed at P18. Overall ANOVA significance was at P < 0.001. There was a significant increase in capillary density from P14 noninjected to both P18 noninjected and PBS-injected (P < 0.001). This increase was not seen in the TA-injected eyes. Both 2 and 4 mg/mL of intravitreous TA resulted in significantly reduced capillary density *P < 0.001 compared with non- and PBS-injected. Numbers inside each bar represent the number of retinas analyzed per group.
Figure 3.
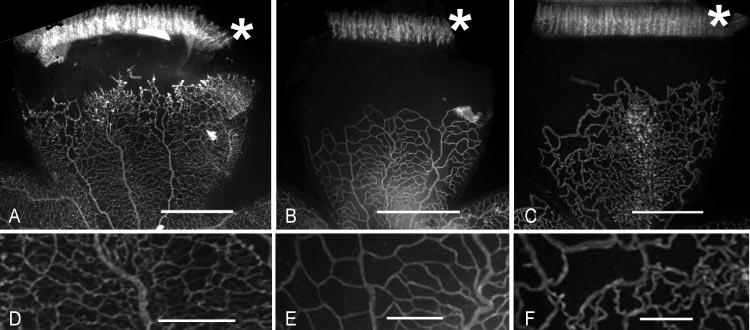
Lectin-stained flatmounted retinas from the 50/10 OIR model after intravitreous injection of TA (doses represent final intravitreous concentrations). ( A) PBS-injected eye; (B) 2 mg/mL TA-injected eye; (C) 4 mg/mL TA-injected eye; (D) higher power image of part of (A); (E) higher-power image of part of (B); (F) higher-power image of part of (C). Retinas in (A), (B), and (C) have the ora serrata attached (marked by ✱). Scale bar: (A–C) 1 mm; (D–F) 0.25 mm. Note the increased NV in (A) compared with (B) and (C) and the lower capillary density in (E) and (F) compared to (D).
Steroid Effect on Weight
We found a dose-dependent decrease in body weight gain at P18 in rat pups that had their eyes injected with TA at P14 compared with those injected with PBS or that had no injection (ANOVA, P = 0.002; Fig. 4).
Figure 4.
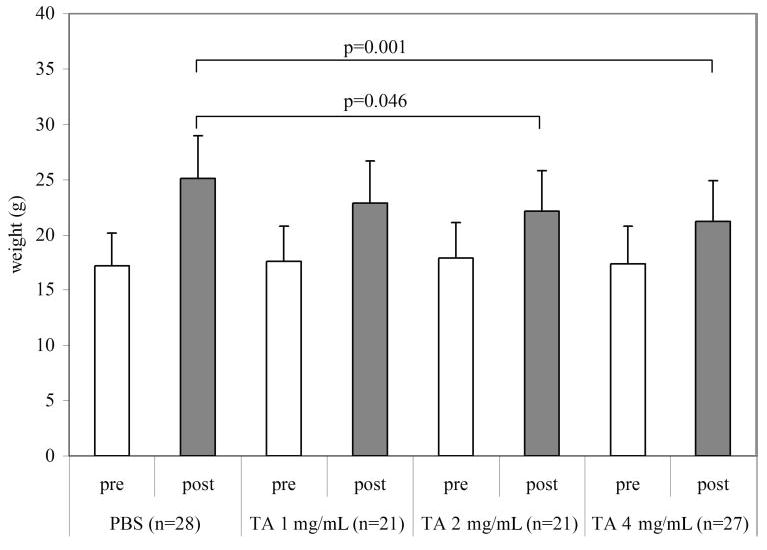
Weight in grams of pups at P14 (before injection [pre]) and at P18 (after injection [post]). Final intravitreous concentrations of TA: 1, 2, and 4 mg. P14 pups were not significantly different from each other, and all gained weight from P14 to P18. However, those animals injected with 2 and 4 mg/mL TA gained significantly less weight than those injected with PBS (overall ANOVA P = 0.002).
Steroid Effect on IGF-1 Signaling
Because corticosteroids have been shown to reduce weight gain in premature infants30 and rats31 and to be associated with suppression of the growth hormone/insulin-like growth hormone signaling pathway,32–34 we wanted to see whether the IGF-1 pathway was being affected by TA-injection. We therefore measured the expression and phosphorylation of the IGF-1 receptor in the retinas of P15 OIR rats that were TA-injected, PBS-injected, or noninjected at P14, and in retinas of P15 control room air–raised rats. We found a trend toward reduced IGF1-R phosphorylation in retinas from TA-injected eyes (4 mg/mL intravitreous concentration) compared with those from PBS- and noninjected eyes of rats in the OIR model or in room air at P15 (Fig. 5).
Figure 5.
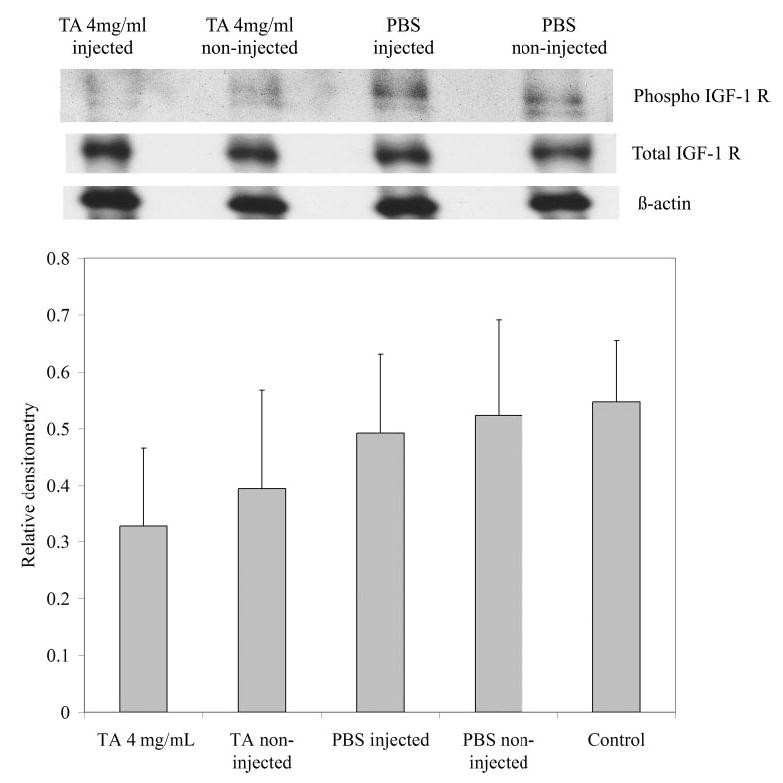
Western blot results. (A) Blots of total IGF-1 receptor, phosphorylated IGF-1 receptor, and β-actin are representative of five retinas from two litters. (B) Bars represent the ratio of phosphorylated IGF-1 receptor to total IGF-1 receptor from TA-injected or non-injected, PBS-injected or non-injected, or control P15 room air–raised rat retinas.
Steroid Effect on Retinal VEGF Expression
Corticosteroids have been shown to reduce VEGF expression through several mechanisms.10,11,35 Therefore, we measured the VEGF expression in rat retinas from PBS-injected, noninjected, and TA-injected eyes at P18 when NV is at its maximum and VEGF has been shown to be upregulated.36 There was no difference in total VEGF protein between groups as measured by ELISA (Fig. 6).
Figure 6.
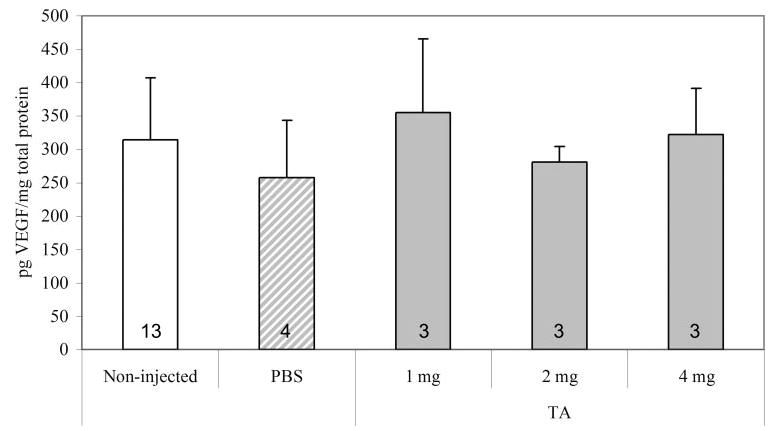
VEGF protein measured by ELISA in retinas from 50/10 OIR model after injections with TA (final intravitreous concentrations, 1, 2, and 4 mg), or PBS, or no injection. There was no significant difference between retinal VEGF levels after injection with TA. Numbers inside each bar represent number of retinas analyzed per group.
Effect of TA on Retinal Apoptosis
Because TA has been reported to be toxic to retinal cells in vitro16,37 and because glucocorticoids have been shown to induce apoptosis in a variety of cells,37 we double-stained retinal flatmounts from TA-injected, PBS-injected, and noninjected eyes with lectin and activated caspase-3 to measure apoptosis. Activated caspase-3 cells were found. However, based on location outside the lectin-stained vasculature, we believe these to be inflammatory cells and not ECs. There also was no difference in the number of apoptotic cells found within the plane of the inner capillary plexus in TA-injected at 1, 2, or 4 mg/mL compared with PBS-injected or noninjected groups (data not shown).
We tested the effect of different doses of TA on human retinal microvascular ECs in culture. After exposure to washed TA for 24 hours, we found a dose-dependent increase in EC death (Fig. 7; overall ANOVA P < 0.001), and virtually all ECs died at the 2000-μg/mL dose. When we stained for activated caspase-3 in TA-exposed cells we could not demonstrate apoptosis (data not shown); however, staurosporine induced apoptosis in these cells (data not shown). These results suggest toxicity of TA at higher doses, though not by induction of apoptosis.
Figure 7.
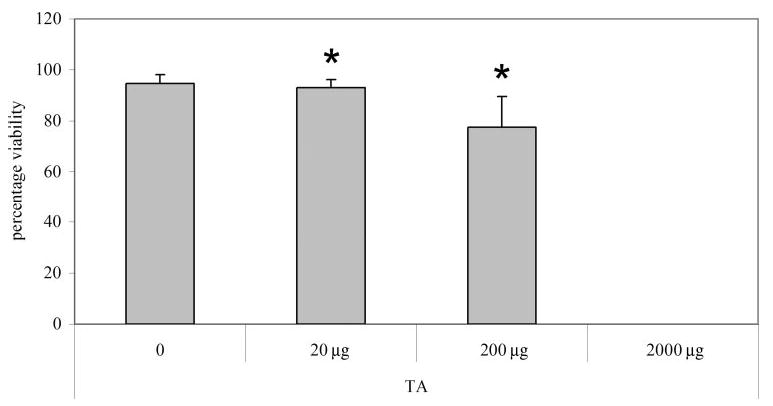
Retinal microvascular cell viability after treatment with increasing doses of TA. Cell viability decreased with increasing TA; at 2000 μg/mL there were no cells counted. Overall ANOVA P < 0.001; *P < 0.001 compared to control. At 200 μg/mL dose (one fifth the intra-vitreous dose of human adult), the cell count was below plating density (data not shown). n = 9 for each group.
Discussion
There is increasing evidence that inflammation plays an important role in the pathogenesis of retinovascular diseases.4,5,38 Clinical trials are under way to test the effect of preservative-free formulations of TA on macular edema associated with diabetes and retinal vein occlusions13 and smaller studies have assessed the effects on NV in ROP (Tawansy KA, et al. IOVS 2005;46:ARVO E-Abstract 3493). Yet, the mechanisms of action of intravitreous steroids are incompletely understood, and concerns have been raised about retinal toxicity because of recent in vitro studies.16,22 Our study was designed to test intravitreous steroid preparations on clock hours of NV in a well-accepted animal model with features most like that of human ROP. We found that TA reduced NV in a dose-dependent manner starting at 1 mg/mL vitreous concentration, whereas DEX did not reduce NV. This was despite the fact that the DEX dose was chosen to achieve the glucocorticoid inhibitory effect of 1.43 mg/mL TA. Other investigators have reported reduced NV from intravitreous TA in a mouse model of oxygen induced vaso-obliteration and NV12 However, the mouse model does not mimic human ROP. In contrast, the rat model achieves a pathology that relates more closely to ROP, with delayed retinal vascularization, peripheral avascular zones adjacent to vascularized retina, and NV at the junction.17 In addition, it does this by exposing rat pups to oxygen extremes similar to as those experienced by a premature infant who developed severe ROP under today’s neonatal intensive care unit (NICU) standards.18
Besides the benefit of reduced NV, we also found reduced capillary density within the vascularized retina of TA-injected compared with PBS- or noninjected eyes. This appeared to be secondary to a delay in vascularization that would occur naturally in the 50/10 OIR model when the rat pups are removed from oxygen cycling and placed in room air.
To determine a reason for the reduced NV and capillary density in TA-injected eyes, we studied the effects of TA on the expression of VEGF, an important angiogenic factor in OIR models36 and in retinal vascular disease.39 TA has been reported to reduce the effects of VEGF through destabilization of its mRNA11 and by reducing IL-β-induced expression.35 However, we did not find a difference in VEGF protein by ELISA. We measured this at P18, a time point when VEGF protein is reported to be upregulated.36 However, it may have been that upregulation of VEGF protein within small regions of the retina had been diluted in measuring VEGF from retinal whole-mounts.
Because we found a dose-dependent decrease in weight gain in rat pups from TA injection, we wanted to determine whether the IGF-1 pathway was being suppressed by the corticosteroid. Corticosteroids have been shown to reduce systemic weight gain in premature infants30 and to reduce systemic IGF-1 levels.32–34 IGF-1 is important in infant growth and retinal vascular development, and its importance in normal retinal vascular development in preterm infants and the subsequent risk of severe ROP has been studied.40,41 We found that signaling through IGF-1 was reduced in the TA-injected group as seen by a trend toward reduction in IGF-1R phosphorylation in the TA-injected group (4 mg/mL) compared with PBS- or noninjected groups in the OIR model and to a room air control group. Our data provide support that IGF-1 signaling is likely reduced by TA. Further study is needed to determine what role IGF-1 plays in the regulation of retinal vascularization and pathologic NV in this OIR model.
Recently, Narayanan et al.16 found reduced viability of two different retinal cell lines in vitro after exposure to 200 μg/mL of TA, which is one fifth the concentration in the vitreous after an intravitreous injection of 4 mg TA, currently being tested for diabetic macular edema or edema associated with retinal vein occlusion.13 Glucocorticoids have also been shown to induce apoptosis in a variety of cells, including lymphocytes, skeletal muscle, chondrocytes, epithelial cells, and corneal epithe-lium.37 We found that TA was toxic to human RECs in vitro in a dose-dependent manner and observed near-total cell death when using concentrations similar to that used clinically. However, this was not through inducing REC apoptosis. In vivo, we found apoptotic cells within retinal wholemounts of all groups. However the apoptotic cells did not stain with lectin and were not associated with vessels, providing evidence that they were not ECs. Nor was there a difference quantitatively in the number of apoptotic cells between TA-injected and PBS-injected, or noninjected eyes. We have previously described apoptosis in rat retinal flatmounts during normal development and with intravitreous injections.42 In addition, apoptosis has been described in other rat OIR models in which animals were exposed to much higher levels of inspired oxygen.43 That we did not find apoptosis of ECs may be, among many reasons, because the vitreous sequestered TA particles from retinal cells. Because TA can have activity within the vitreous for more than a month,44–46 long-term study should be considered. Similar to the study of Narayanan et al.,16 we found that TA’s effect was independent of the vehicle in vivo.
We found a statistically significant reduction in systemic weight gain from intravitreous injections of TA. Another group assayed the serum of adults who received approximately 5 to 6.25 mg/mL intravitreous concentrations of TA, higher concentrations than those in our study. By high-performance liquid chromatography, this group found negligible TA in the serum of 20 patients, most samples having undetectable levels and the highest being 0.8 μg/L.47 In neonates, 0.5 mg/kg of dexamethasone (equivalent to 40 μg/mL TA in a 2.5-kg premature infant) administered intravenously for 3 days followed by a tapering dose for 12 days was associated with early initial weight loss.30 This systemic dose would be greater than that expected in a 2.5-kg premature infant with a 1-mg/mL intravitreous concentration of TA after taking into account the approximate 30-fold difference in blood volumes between premature infants and adults. If we assumed a choroidal concentration equivalent to that of the vitreous,48 the systemic concentration of TA in a 16-g rat pup with a 1-mg/mL intravitreous concentration of TA would be approximately 15 μg/mL lower than the systemic concentration in the pediatric study,30 but still higher than the extrapolated dose in a 2.5-kg premature infant. Although a direct comparison between the premature human infant and a rat pup cannot be made, our data suggest that further study be made before considering TA doses in premature infants, particularly because TA can remain within the vitreous for extended periods.44–46
Our study shows that TA reduces NV and intraretinal vascularization in a model of OIR with the new finding that this appears to be associated with reduced phosphorylation of the IGF-1 receptor. We did not find reduced VEGF expression or apoptosis of EC as a mechanism in vivo, but recognize that further studies would be needed to exclude these possibilities. Although there is a possibility of a crossover effect in the fellow non-injected eye, we did not find this. Furthermore, we saw no difference in PBS versus non-injected eyes. Our data support those of previous investigators that further study of the mechanisms and safety of TA injected into the vitreous be performed particularly before considering TA as a treatment for diseases where nascent intraretinal vascularization is ongoing, such as ROP. It also serves as a reminder that consideration be given to the differences in blood volumes between the adult human and premature infant when choosing intravitreous doses of drugs.
Acknowledgments
The authors thank Stephen Aylward, PhD, and Zheen Zhao, University of North Carolina’s Computer Aided Diagnosis and Display Laboratory, Department of Radiology, for assistance with image analysis and Gerard Lutty, PhD, for the kind gift of retinal microvascular ECs.
Footnotes
Disclosure: M.E. Hartnett, None; D.J. Martiniuk, None; Y. Saito, None; P. Geisen, None; L.J. Peterson, None; J.R. McColm, None
Supported by National Eye Institute Grant R01 EY015130 and Research to Prevent Blindness.
References
- 1.Funatsu H, Yamashita H, Shimizu E, Kojima R, Hori S. Relationship between vascular endothelial growth factor and interleukin-6 in diabetic retinopathy. Retina. 2001;21:469–477. doi: 10.1097/00006982-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and inter-leukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong D, Ueda T, Aljeda A, et al. Lipid hydroperoxide stimulates retinal neovascularization in rabbit retina through expression of TNF-alpha, VEGF, and PDGF. Angiogenesis. 1998;2:93–104. doi: 10.1023/a:1009010628371. [DOI] [PubMed] [Google Scholar]
- 4.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 5.Ishida S, Yamashiro K, Usui T, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–789. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 6.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–430. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young TL, Anthony DC, Pierce E, Foley E, Smith LEH. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS. 1997;1:105–110. doi: 10.1016/s1091-8531(97)90008-2. [DOI] [PubMed] [Google Scholar]
- 8.Usui T, Ishida S, Yamashiro K, et al. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci. 2004;45:368–374. doi: 10.1167/iovs.03-0106. [DOI] [PubMed] [Google Scholar]
- 9.Graham EM, Stanford MR, Shilling JS, Sanders MD. Neovascularisation associated with posterior uveitis. Br J Ophthalmol. 1987;71:826–833. doi: 10.1136/bjo.71.11.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuda S, Gomi F, Oshima Y, Tohyama M, Tano Y. Vascular endothelial growth factor reduced and connective tissue growth factor induced by triamcinolone in ARPE19 cells under oxidative stress. Invest Ophthalmol Vis Sci. 2005;46:1062–1068. doi: 10.1167/iovs.04-0761. [DOI] [PubMed] [Google Scholar]
- 11.Sears JE, Hoppe G. Triamcinolone acetonide destabilizes VEGF mRNA in Muller cells under continuous cobalt stimulation. Invest Ophthalmol Vis Sci. 2005;46:4336–4341. doi: 10.1167/iovs.05-0565. [DOI] [PubMed] [Google Scholar]
- 12.Spandau UHM, Sauder G, Schubert U, Hammes HP, Jonas JB. Effect of triamcinolone acetonide on proliferation of retinal endothelial cells in vitro and in vivo. Br J Ophthalmol. 2005;89:745–747. doi: 10.1136/bjo.2004.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott IU, Ip MS. It’s time for a clinical trial to investigate intravitreal triamcinolone for macular edema due to retinal vein occlusion: The SCORE study. Arch Ophthalmol. 2005;123:581–582. doi: 10.1001/archopht.123.4.581. [DOI] [PubMed] [Google Scholar]
- 14.Jonas JB, Kreissig I, Degenring R. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog Retin Eye Res. 2005;24:587–611. doi: 10.1016/j.preteyeres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2003;110:1517–1525. doi: 10.1016/S0161-6420(03)00544-X. [DOI] [PubMed] [Google Scholar]
- 16.Narayanan R, Mungcal JK, Kenney MC, Seigel GM, Kuppermann BD. Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:722–728. doi: 10.1167/iovs.05-0772. [DOI] [PubMed] [Google Scholar]
- 17.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 19.Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004;122:1131–1136. doi: 10.1001/archopht.122.8.1131. [DOI] [PubMed] [Google Scholar]
- 20.Majji AB, Jalali S, Das T, Gopinathan U. Role of intravitreal dexamethasone in exogenous fungal endophthalmitis. Eye. 1999;13:660–665. doi: 10.1038/eye.1999.179. [DOI] [PubMed] [Google Scholar]
- 21.Berkowitz BA, Lukaszew RA, Mullins CM, Penn JS. Impaired hyaloidal circulation function and uncoordinated ocular growth patterns in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1998;39:391–396. [PubMed] [Google Scholar]
- 22.Morrison VL, Koh HJ, Cheng L, Bessho K, Davidson MC, Freeman WR. Intravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rabbits. Retina. 2006:339–344. doi: 10.1097/00006982-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Arumi J, Boixadera A, Giralt J, et al. Comparison of different techniques for purification of triamcinolone acetonide suspension for intravitreal use. Br J Ophthalmol. 2005;89:1112–1114. doi: 10.1136/bjo.2005.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spandau UHM, Derse M, Schmitz-Valckenberg P, Papoulis C, Jonas JB. Dosage dependency of intravitreal triamcinolone acetonide as treatment for diabetic macular oedema. Br J Ophthalmol. 2005;89:999–1003. doi: 10.1136/bjo.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995;36:2063–2070. [PubMed] [Google Scholar]
- 26.Chan-Ling T. Glial, vascular and neuronal cytogenesis in whole-mounted cat retina. Microsc Res Tech. 1997;36:1–16. doi: 10.1002/(SICI)1097-0029(19970101)36:1<1::AID-JEMT1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 27.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Leske DA, Holmes JM. Neovascularization grading methods in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2000;41:887–891. [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Halliday HL, Patterson CC, Halahakoon CWNL on Behalf of the European Multicenter Steroid Study Group. A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics. 2001;107:232–240. doi: 10.1542/peds.107.2.232. [DOI] [PubMed] [Google Scholar]
- 31.Viires N, Pavlovic D, Pariente R, Aubier M. Effects of steroids on diaphragmatic function in rats. Am Rev Respir Dis. 1990;142:34–38. doi: 10.1164/ajrccm/142.1.34. [DOI] [PubMed] [Google Scholar]
- 32.Huysman MWA, Hpkken-Koelega ACS, Hop WCJ, Sauer PJJ. Effect of dexamethasone treatment on serum GH, IGF-I, and the binding proteins IGFBP-1 and -3 in ventilated very preterm infants. Pediatr Res. 2003;54:37–43. doi: 10.1203/01.PDR.0000065727.45195.69. [DOI] [PubMed] [Google Scholar]
- 33.Bloomfield FH, Knight DB, Breier BH, Harding JE. Growth restriction in dexamethasone-treated preterm infants may be mediated by reduced IGF-I and IGFBP-3 plasma concentrations. Clin Endocrinol. 2001;54:235–242. doi: 10.1046/j.1365-2265.2001.01219.x. [DOI] [PubMed] [Google Scholar]
- 34.Skinner AM, Battin M, Solimano A, Daaboul J, Kitson HF. Growth and growth factors in premature infants receiving dexamethasone for bronchopulmonary dysplasia. Am J Perinatol. 1997;14:539–546. doi: 10.1055/s-2007-994330. [DOI] [PubMed] [Google Scholar]
- 35.Itakura H, Akiyama H, Hagimura N, et al. Triamcinolone acetonide suppresses interleukin-1 beta-mediated increase in vascular endothelial growth factor expression in cultured rat Müller cells. Graefes Arch Clin Exp Ophthalmol. 2005;28:1–6. doi: 10.1007/s00417-005-0052-1. [DOI] [PubMed] [Google Scholar]
- 36.Werdich XQ, McCollum GW, Rajaratnam VS, Penn JS. Variable oxygen and retinal VEGF levels: correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Exp Eye Res. 2004;79:623–630. doi: 10.1016/j.exer.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Bourcier T, Forgez P, Borderie V, Scheer S, Rostene W, Laroche L. Regulation of human corneal epithelial cell proliferation and apoptosis by dexamethasone. Invest Ophthalmol Vis Sci. 2000;41:4133–4141. [PubMed] [Google Scholar]
- 38.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Path. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Eng J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 40.Smith LEH, Kopchick JJ, Chen W, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 41.Hellstrom A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87:3413–6. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 42.McColm JR, Geisen P, Peterson LJ, Hartnett ME. Exogenous leukemia inhibitory factor (LIF) attenuates retinal vascularization. Exp Eye Res. 2006;83:438–446. doi: 10.1016/j.exer.2006.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beauchamp MH, Marrache AM, Hou X, et al. Platelet-activating factor in vasoobliteration of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2002;43:3327–3337. [PubMed] [Google Scholar]
- 44.Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–686. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 45.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and non-vitrectomized eyes. Retina. 2005;25:556–560. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Mason JO3, Somaiya MD, Singh RJ. Intravitreal concentration and clearance of triamcinolone acetonide in nonvitrectomized human eyes. Retina. 2004;24:900–904. doi: 10.1097/00006982-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Degenring RF, Jonas JB. Serum levels of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004;137:1142–1143. doi: 10.1016/j.ajo.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Driot JY, Novack GD, Rittenhouse KD, Milazzo C, Pearson AP. Ocular pharmacokinetics of fluocinolone acetonide after retisert intravitreal implantation in rabbits over a 1-year period. J Ocul Pharmacol Ther. 2004;20:269–275. doi: 10.1089/1080768041223611. [DOI] [PubMed] [Google Scholar]


