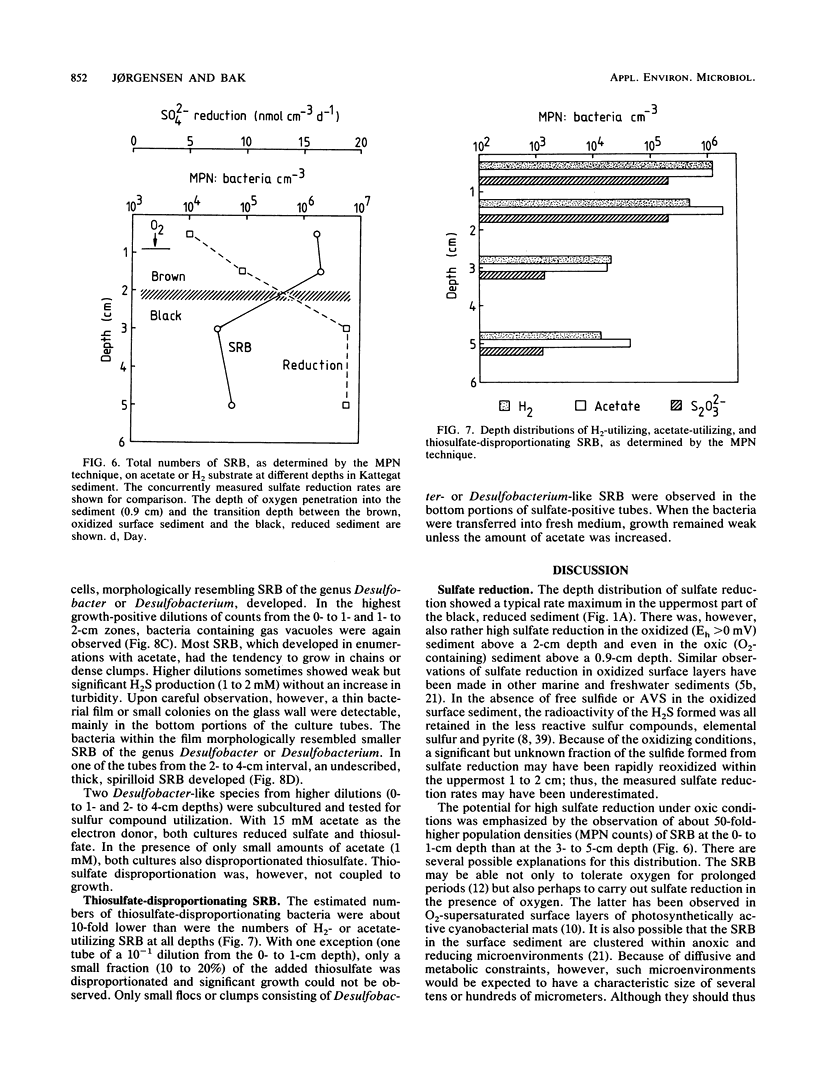
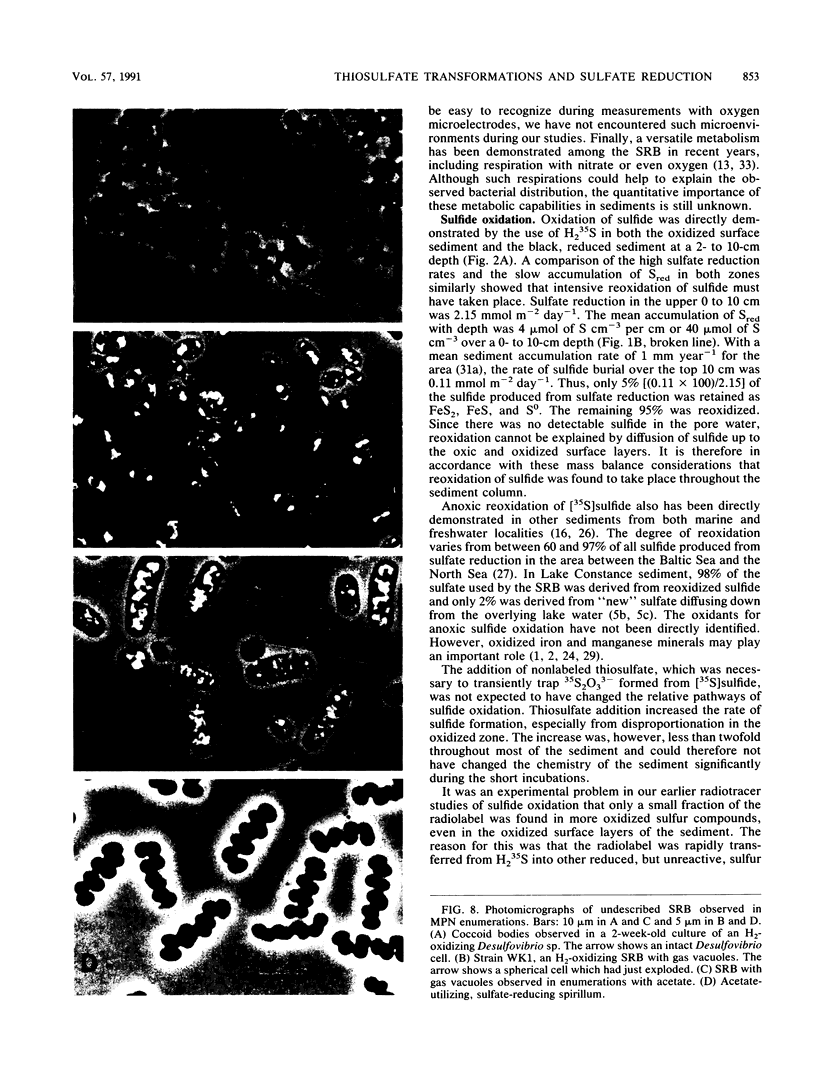
Abstract
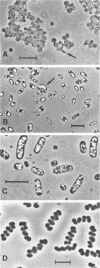
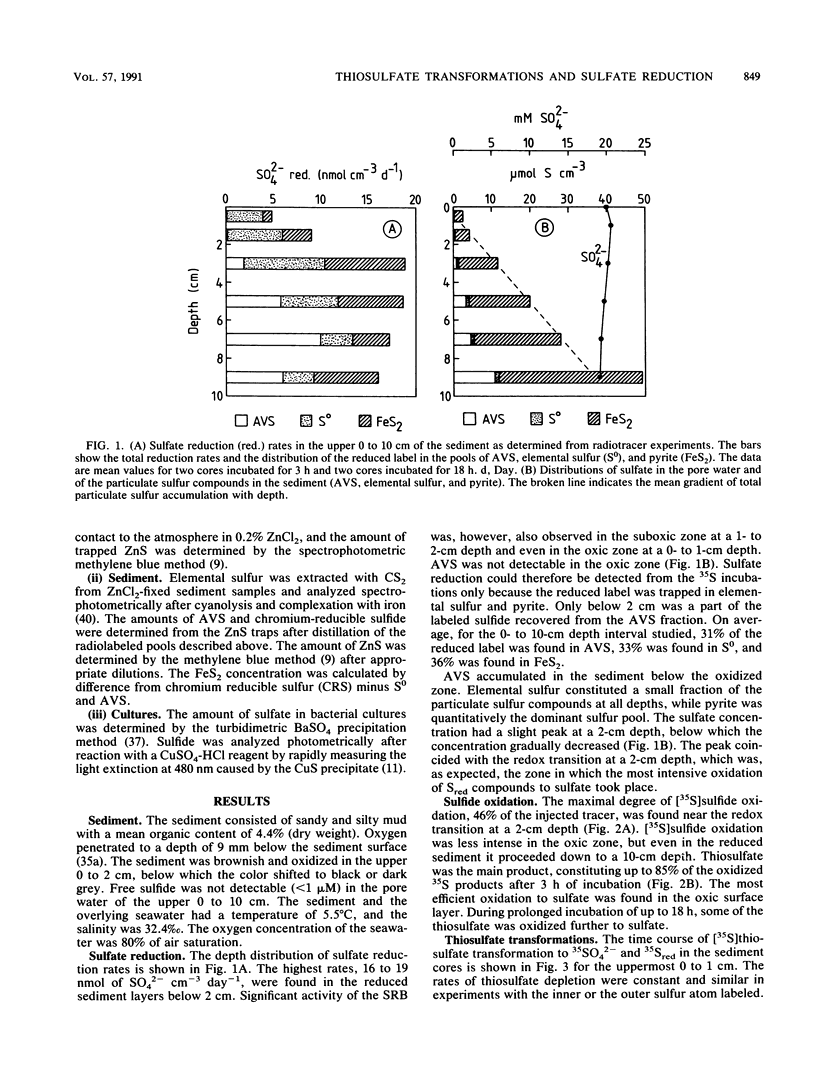
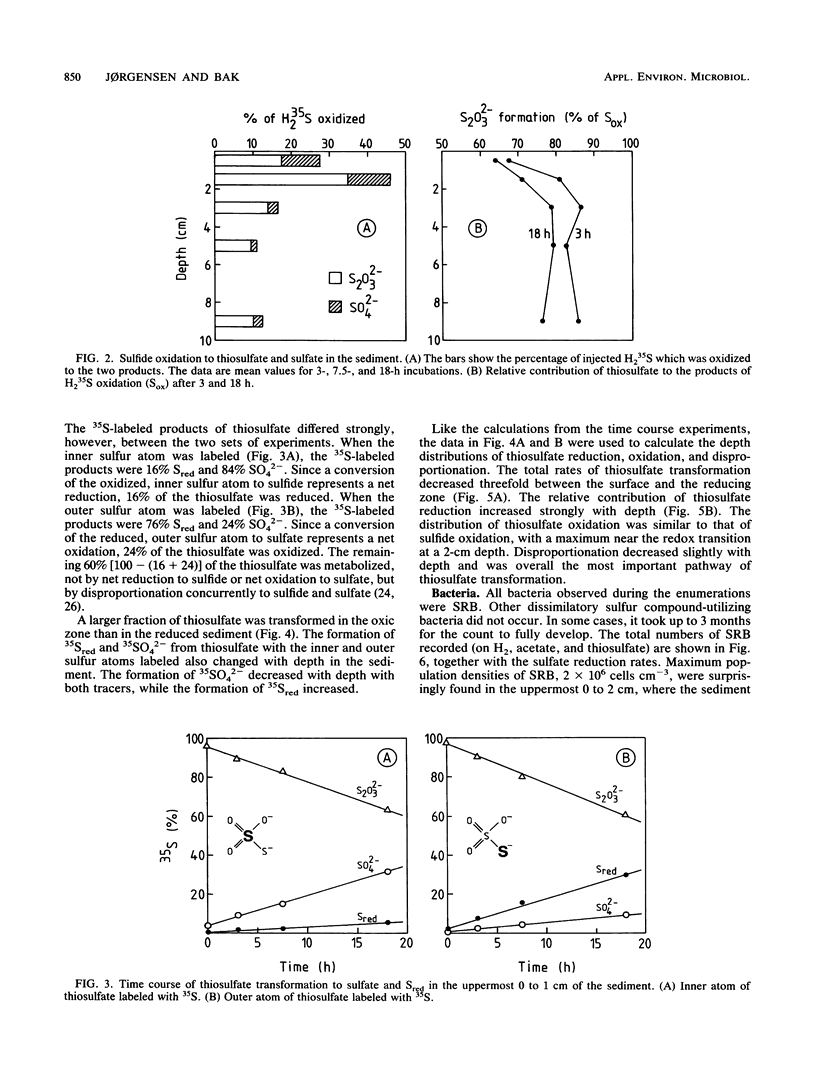
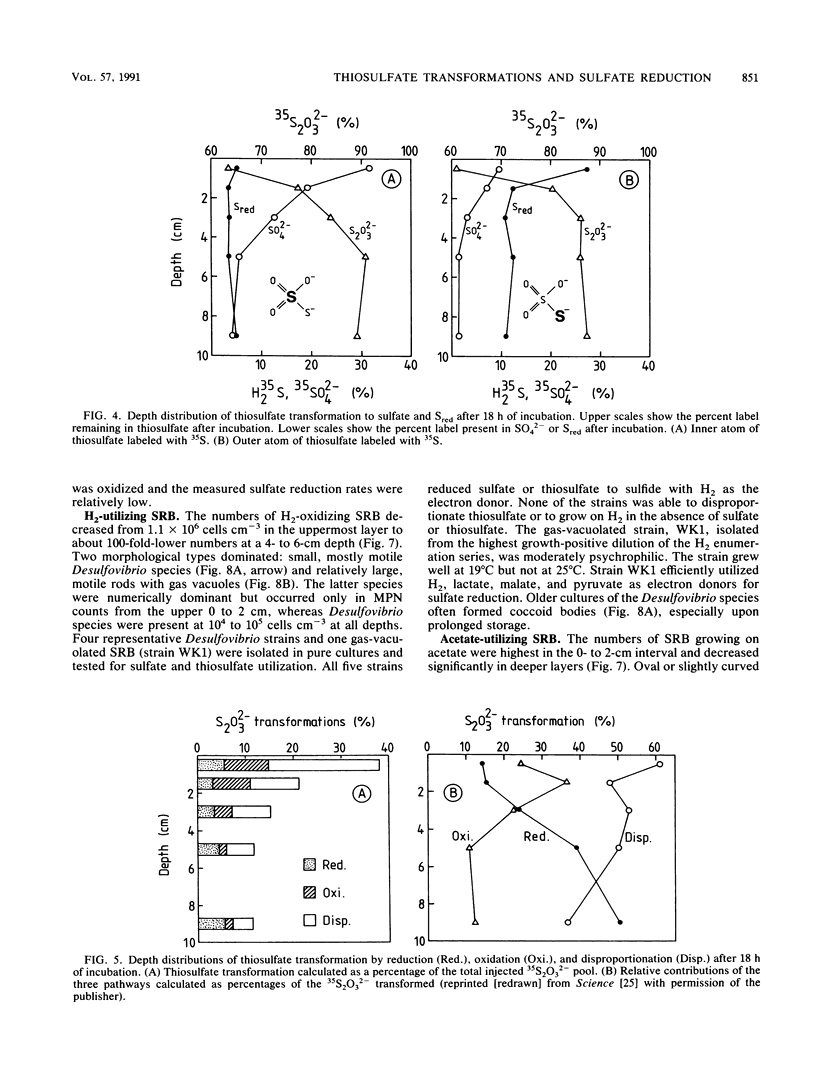
Reductive and oxidative pathways of the sulfur cycle were studied in a marine sediment by parallel radiotracer experiments with 35SO42-, H235S, and 35S2O32- injected into undisturbed sediment cores. The distributions of viable populations of sulfate- and thiosulfate-reducing bacteria and of thiosulfate-disproportionating bacteria were concurrently determined. Sulfate reduction occurred both in the reducing sediment layers and in oxidized and even oxic surface layers. The population density of sulfate-reducing bacteria was >106 cm-3 in the oxic layer, high enough that it could possibly account for the measured rates of sulfate reduction. The bacterial numbers counted in the reducing sediment layers were 100-fold lower. The dominant sulfate reducers growing on acetate or H2 were gas-vacuolated motile rods which were previously undescribed. The products of sulfide oxidation, which took place in both oxidized and reduced sediment layers, were 65 to 85% S2O32- and 35 to 15% SO42-. Thiosulfate was concurrently oxidized to sulfate, reduced to sulfide, and disproportionated to sulfate and sulfide. There was a gradual shift from predominance of oxidation toward predominance of reduction with depth in the sediment. Disproportionation was the most important pathway overall. Thiosulfate disproportionation occurred only as cometabolism in the marine acetate-utilizing sulfate-reducing bacteria, which could not conserve energy for growth from this process alone. Oxidative and reductive cycling of sulfur thus occurred in all sediment layers with an intermediate “thiosulfate shunt” as an important mechanism regulating the electron flow.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banat I. M., Lindström E. B., Nedwell D. B., Balba M. T. Evidence for coexistence of two distinct functional groups of sulfate-reducing bacteria in salt marsh sediment. Appl Environ Microbiol. 1981 Dec;42(6):985–992. doi: 10.1128/aem.42.6.985-992.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen B. B. A thiosulfate shunt in the sulfur cycle of marine sediments. Science. 1990 Jul 13;249(4965):152–154. doi: 10.1126/science.249.4965.152. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Pfennig N. Oxidation of short-chain fatty acids by sulfate-reducing bacteria in freshwater and in marine sediments. Arch Microbiol. 1981 Jan;128(3):330–335. doi: 10.1007/BF00422540. [DOI] [PubMed] [Google Scholar]
- Luther G. W., 3rd, Church T. M., Scudlark J. R., Cosman M. Inorganic and organic sulfur cycling in salt-marsh pore waters. Science. 1986 May 9;232(4751):746–749. doi: 10.1126/science.232.4751.746. [DOI] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F. Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol. 1986 May;51(5):1056–1062. doi: 10.1128/aem.51.5.1056-1062.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Ward D. M. Substrates for sulfate reduction and methane production in intertidal sediments. Appl Environ Microbiol. 1983 Jan;45(1):193–199. doi: 10.1128/aem.45.1.193-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]