Abstract
The newt is one of the few organisms that is able to undergo lens regeneration as an adult. This review will examine the signaling pathways that are involved in this amazing phenomenon. In addition to outlining the current research involved in elucidating the key signaling molecules in lens regeneration, we will also highlight some of the similarities and differences between lens regeneration and development.
Keywords: Regeneration, Newt, Transdifferentiation
Introduction
Regeneration of lost body parts is a remarkable phenomenon that few organisms can undergo. Some salamanders, among them the adult newt, Notophthalmus viridescens, have remarkable regenerative abilities. The newt is capable of regenerating most tissues, one of which is the lens. Lens regeneration occurs through a process of transdifferentiation. In this process, pigmented epithelial cells (PECs) of the dorsal iris dedifferentiate and ultimately differentiate into a completely different cell type to form a lens. While much attention has been paid to the process of lens regeneration in the newt, the exact mechanism by which this feat is accomplished is not yet fully understood. One thing that is apparent, however, is the importance of signal transduction mechanisms in inducing lens regeneration. Most speculation on the signal transduction pathways involved in lens regeneration comes from developmental studies. It is a widely held belief that the signaling and induction events that occur during eye and lens development play similar roles in lens regeneration as both processes accomplish the same feat. In recent years, research has started to delineate some of the signaling involved in lens regeneration. Many of the common signal transduction pathways have not been thoroughly examined during lens regeneration and much more remains to be discovered (Figure 1). The fact that the newt genome is not sequenced has hindered this to some extent, but despite this progress is being made. In this review, we will highlight what is known regarding cell signaling in lens regeneration.
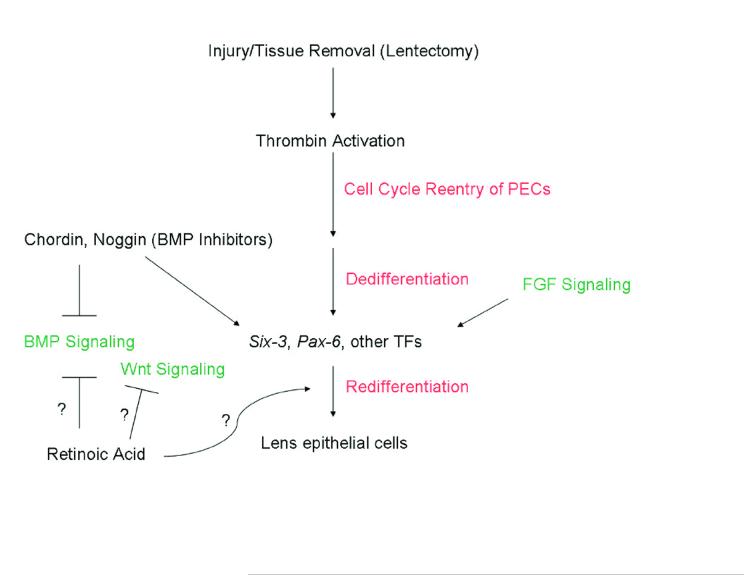
Figure 1.
Outline of events and pathways likely to be involved in lens regeneration. Upon lens removal or injury, PECs must reenter the cell cycle and thrombin is suspected to play a role in this. Following proliferation, the PECs dedifferentiate and then redifferentiate. The BMP, Wnt, and FGF pathways play pivotal roles in lens development. BMP antagonists have been shown to upregulate TFs such as six3 in the newt during regeneration as well as induce lens regeneration. RA has been shown to inhibit BMP and Wnt signaling in other systems, but the mechanism through which it works in inducing (along with six3) newt lens regeneration is unclear. Question marks depict possible roles of RA for induction of lens regeneration.
Process of Lens Regeneration
Following removal of the lens, the regenerative process begins with dedifferentiation of the PECs of the dorsal iris. Dedifferentiation marks the beginning of cell cycle reentry and proliferation. At approximately 10 days post-lentectomy depigmented PECs form a vesicle at the tip of the dorsal iris [1,2]. Cells in the inner layer of the vesicle begin to elongate and differentiate into primary lens fiber cells at 12-16 days post-lentectomy. This time period is also marked by proliferation and crystallin synthesis. At day 15-20 post-lentectomy, primary lens fiber cells continue to grow from the inner layer while cells from the outer layer of the vesicle begin to form secondary fibers. A complete lens is formed 25 days following removal [3,4] .
Thrombin Signaling Pathway
One area of signaling often overlooked in regeneration is the injury response cascade, particularly the coagulation pathway involving thrombin. Injury leads to the activation of thrombin by the release of prothrombin (inactive form) from the vasculature. While injury results in the release of prothrombin, it is the presence of a membrane protein, Tissue Factor (clotting factor III), which is actually responsible for the activation of thrombin [5]. Thrombin is known to play a role in cell cycle re-entry on newt cells. Treatment of newt skeletal myotubes with thrombin induces proliferation, whereas mouse myotubes do not exhibit the same response [6]. This species-specific response led to speculation that thrombin might have an important role in the cell cycle re-entry of postmitotic cells known to occur in regenerating tissues. This speculation was further supported by Simon and Brockes when they showed that pigmented epithelial cells (PECs), both dorsal and ventral, of adult newt iris could be stimulated to reenter S-phase of mitosis with thrombin treatment in vitro [7]. Perhaps the strongest support for a role of thrombin in lens regeneration came from Imokawa and Brockes when they were able to show thrombin activation at the pupillary margin of the dorsal iris during lens regeneration in the newt from 20 minutes post-lentectomy until about 7 days post-lentectomy [8]. No thrombin activation was seen in the regeneration incompetent ventral iris at any stage [8]. Importantly, inhibition of thrombin led to a loss of cell cycle re-entry at the dorsal margin and inhibition of or incomplete lens regeneration [8,9]. The final support for a role of thrombin signaling in lens regeneration comes from the axolotl, another urodele capable of limb, but not lens, regeneration. Activated thrombin was detected in the mesenchymal tissue of the blastema during limb regeneration, but not seen at any stage in the iris following lentectomy. Future studies on the expression pattern of Tissue Factor, the activator of prothrombin, will be important to clarify this issue. Based on their findings, Imokawa and Brockes suggest that Tissue Factor should be expressed in the dorsal margin of the newt during regeneration and absent in the axolotl iris [8,9]. These studies provide strong evidence for a role of thrombin in postmitotic cell cycle reentry of newt iris PECs both in vitro and in vivo.
FGF Signaling Pathway
Fibroblast growth factors and their receptors are critical for various stages of lens development, including induction, proliferation, and differentiation. FGFs play a dominant role in initiating fiber differentiation and regulating the spatial and temporal pattern of crystallin gene expression [10,11]. In chicks, ectopic FGF-8 expression in the distal optic vesicle leads to the expansion of the lens field [12]. Targeted overexpression of FGFs in lenses of transgenic mice leads to inappropriate proliferation and differentiation of the lens epithelium [13-15].
Several of the FGF ligands have also been found to be involved in lens regeneration. Del Rio-Tsonis and colleagues were the first to show the production of a second lens after FGF4 treatment in lentectomized eyes. Treatment of lentectomized newts with both FGF1 and FGF4 induced lenses with abnormal polarity due to differentiation of lens epithelial cells to lens fibers, and double lens formation from the dorsal iris [16]. These abnormalities in the regenerating lenses appeared to be similar to the lens polarity abnormalities induced during lens development in transgenic FGF mice [17]. FGF2 and FGF4 have been shown to be essential for in vitro lens regeneration from the pigmented cells of the dorsal iris [18]. Dorsal iris cellular reaggregates cultured on collagen coated dishes developed lenses in vitro only when treated with FGF2/4 [18]. Hayashi and colleagues have shown that intraocular injection of recombinant FGF2 can trigger lens regeneration from the dorsal iris without previously removing the host lens. Injection of FGF2 also induced expression of several transcription factors such as Pax6, Sox2, and MafB [19]. Intraocular injections of recombinant newt FGF1 also had effects on newt lens regeneration [20]. In these experiments, the injection of FGF1 caused depigmentation and dedifferentiation of both the dorsal and ventral irises with lens like structures forming from the dorsal irises, albeit with a very thin or missing lens epithelium and abnormal lens fiber cells [20].
Studies on lens regeneration in another organism, Xenopus laevis, have also implicated a role of FGFs in lens regeneration. Xenopus larvae are capable of regenerating a lens from the outer cornea after lentectomy [21]. When isolated Xenopus larvae outer corneas were cultured in the presence of FGF-1, differentiation of the cornea to lens fibers was observed [22].
Past studies suggest that different FGF receptors play significant roles during lens regeneration. Several FGF receptors are expressed including FGFR1, FGFR2, and FGFR3 [16,23,24]. Detailed examination of the receptors, showed that FGFR-1 is confined to the dedifferentiating dorsal iris and plays a role in regulating lens regeneration. McDevitt and others showed that lens regeneration could be interrupted by injecting an FGF receptor-directed mitotoxin into regenerating newt eyes [24]. In another inhibition study, newts were maintained in a solution of SU5402, a chemical that prevents autophosphorylation of FGFR-1[23]. This treatment inhibited lens regeneration and fiber differentiation, suggesting that FGFR-1 signaling plays a key role in lens regeneration [23]. SU5402 also inhibited lens regeneration of cellular aggregates consisting of dorsal PECs that were treated with it [18]. However, transfection of FGFR-1 into cultured newt iris PECs (dorsal and ventral) with subsequent re-implantation into host newts did not induce lens regeneration from the ventral iris (0/11 – 0%) and had no observable effect on lens regeneration from the dorsal iris (2/7 – 29%) (Tsonis lab; unpublished) suggesting that FGFR-1 signaling is not sufficient for lens regeneration. To confirm that FGF activity is essential to lens regeneration, Hayashi and others also injected soluble FGF receptors to compete with endogenous receptors for FGF binding [19]. It was observed during FGFR2(IIIc)/Fc (binding FGF 1,2,4, and 9) daily injections that lens regeneration was completely inhibited. However, when FGFR2(IIIb)/Fc (binding FGF 1,7, and 10) was injected, no effect on lens regeneration was observed [19].
BMP Signaling Pathway
The BMP signaling pathway, particularly via BMP4 and BMP7, plays important roles in development of the vertebrate eye and lens [25-31]. Much less is known about BMP signaling during lens regeneration. This is partly due to the fact that lens regeneration occurs in very few vertebrates and partly due to the fact that the newt genome is not yet sequenced and thus the expression of many players in the newt BMP pathway have not been documented. However, we have recently shown that BMPs play important functional roles in lens regeneration. We reported that lens regeneration can be induced from the regeneration incompetent ventral iris with two separate BMP inhibition treatments [32]. In this study newt iris explants (dorsal and ventral) were treated with recombinant mouse chordin protein or a truncated, signaling incompetent form of human BMPR-IA to inhibit BMP signaling and then re-implanted into a host newt to observe any effect on lens regeneration. In both treatments lens regeneration was induced from the normally incompetent ventral iris. Treatment with BMP ligands yielded further support for BMP signaling playing a role in lens regeneration. Consistent with inhibition of BMPs inducing lens regeneration from the normally incompetent ventral iris, dorsal iris explants treated with either BMP4 or BMP7 showed a decrease in lens regeneration. In vivo expression of BMPR-IA was examined and it was found that BMPR-IA is expressed in both the dorsal and ventral intact iris and during all stages of lens regeneration. While BMP signaling appears to play an important role in the process of lens regeneration, much research is needed to determine the key players in this pathway and how BMP signaling is regulated during lens regeneration.
Wnt Signaling Pathway
The canonical Wnt signaling pathway plays an important role during several stages of eye development including eye field specification and lens differentiation [33]. An important aspect to both lens development and lens regeneration is the differentiation of lens fiber cells. In the case of lens development, the Wnt/β-catenin signaling pathway appears essential for the differentiation of the lens epithelium into lens fiber cells [34-36]. Mice with a homozygous null mutation in the lrp6 co-receptor gene are unable to fully undergo differentiation of the lens epithelium [35]. In addition, Wnt signaling causes an increase in β-crystallin, a marker of fiber cell differentiation, in lens epithelial cells. These same lens epithelial cells when primed with FGF-2 results in elongation of the lens epithelial cells in addition to β-crystallin accumulation [34]. In recent years, another set of regulatory molecules of the canonical Wnt signaling pathway, Dickkopfs (Dkks), has been intensely studied for its role in lens development. During lens development Dkk1, Dkk2, and Dkk3 are expressed in a pattern similar to that seen for Wnts and is expressed mainly in the epithelium [37,38]. Dickkopfs antagonize the pathway by binding to the LRP co-receptor and preventing the stabilization of the β-catenin complex. A study examining β-catenin function in lens development found that loss of β-catenin function in the periocular ectoderm of embryonic mice results in ectopic lentoid body formation [36].
Based on the role of the Wnt/β-catenin pathway in lens development it seems logical that this pathway may also play an important role in lens regeneration. While recent work has shown the importance of Wnt signaling in lens development, little work has been done examining this pathway's role in the process of lens regeneration. In order to examine the potential role of Wnt/β-catenin signaling during lens regeneration, we utilized a GSK-3β inhibitor as well as small-molecule antagonists of the Tcf/β-catenin protein complex [39,40]. While these molecules will not conclusively show the role of Wnt signaling in lens regeneration due to the multiple roles of β-catenin and the ability of Wnt to signal through multiple pathways, it will provide some initial insight into the role of β-catenin in lens regeneration. In both cases, dorsal and ventral iris explants were treated in culture and subsequently implanted into lentectomized newt eyes. Neither activation nor inhibition of Wnt signaling by these treatments affected lens regeneration (Tsonis lab unpublished data) suggesting that β-catenin signaling does not play a role during lens regeneration. Further work must be done to determine the role of β-catenin and the different Wnt signaling pathways in lens regeneration.
Transcription Factors
Transcription factors are important molecules for mediating the downstream events of signaling cascades involved in many processes including lens development and regeneration. Two transcription factors, which are involved in both lens development and regeneration, are Pax-6 and Six-3.
Pax-6
Pax-6 has long been considered the “master eye gene” due to its necessity and sufficiency for lens formation. It is thought to sit at the top of a hierarchy of genes regulating lens development. Heterozygous mutations in Pax-6 lead to aniridia in humans and the “small eye” phenotype in mice [41,42]. It has also been shown that overexpression of Pax-6 induces the formation of ectopic eyes in Drosophila and Xenopus [43,44]. During lens regeneration, Pax-6 is expressed in both the dorsal and ventral iris PECs. As regeneration continues, expression of Pax-6 localizes to the dorsal iris and eventually becomes restricted to the regenerating lens epithelium [45]. Recent evidence suggests that Pax-6 is not essential for the induction of lens regeneration. Contrary to its early role in embryonic lens specification and induction, Pax-6 appears to play a role during the later stages of regeneration. One line of evidence that supports this notion is that overexpression of Pax-6 in newt ventral iris PECs did not induce transdifferentiation of the ventral iris into lens [32]. Further evidence pointing to a role of Pax-6 in later events of lens regeneration such as proliferation of both the dorsal and ventral iris PECs and regulation of crystallin synthesis has been shown through the use of morpholino technology. In these experiments, newt iris cells treated with a Pax-6 morpholino had a decreased rate of proliferation in the iris PECs both in vitro and in vivo and as a result lens regeneration was significantly retarded. However, dedifferentiation of the dorsal iris was not inhibited. Treatment with the morpholino at early stages of lens regeneration resulted in a decrease in crystallin expression and slowing of lens fiber induction. Crystallin synthesis and lens fiber maintenance were not affected upon treatment with the Pax-6 morpholino after these processes had already ensued [46].
Six-3
Six-3 is another important transcription factor necessary for lens formation. Six-3 is a member of the six-homeodomain family and is expressed in the lens placode and eventually in the lens epithelium at later stages of lens development [47]. Misexpression of Six-3 in the 2-4 cell stage medaka embryos results in the formation of ectopic lenses within the otic placode [48]. Other evidence lending support to the role of Six-3 in lens induction comes from studies by Carl et al., (2002) in which morpholino knock-down of Six-3 expression in the two-cell stage of medaka embryos resulted in absence of forebrain and eyes [49]. These data suggest that Six-3, along with Pax-6, is also at the top of the hierarchy of genes that is responsible for lens induction. Indeed, it has been shown that Six-3 and Pax-6 can mutually activate one another through binding sites in their respective enhancer elements [50].
In intact newt iris tissue, Six-3 is expressed in both the dorsal and ventral iris with a higher concentration in the ventral tissue. However, during regeneration only the dorsal iris shows a significant increase in Six-3 expression. In addition, treatment of ventral iris PECs with Six-3/retinoic acid induced transdifferentiation of the lens regeneration incompetent ventral iris [32]. Importantly, Six-3 alone did not cause transdifferentiation of ventral iris PECs but required the presence of retinoic acid (RA). The role of RA will be discussed below. Induction of lens regeneration from the ventral iris by six-3/RA shows the importance of Six-3 in inducing lens regeneration. Perhaps it is the increase in Six-3 expression above the normal threshold levels for both the dorsal and ventral iris that is the key to inducing lens regeneration as opposed to the relative amount of Six-3. Treatment of ventral iris PECs with Six-3/RA causes the ventral iris tissue to adopt an expression profile similar to that seen in the dorsal iris during lens regeneration [32]. In relation to the BMP pathway, we have shown that treatment of newt ventral iris tissue with chordin increases the expression of Six-3 and Pax-6 [32]. These results are consistent with a developmental study on eye field specification in Xenopus in which another BMP inhibitor, noggin, sits atop the pathway and leads to increased expression of Six-3, Pax-6 and other eye transcription factors [51]. These data also support the notion of Six-3 being near the top of the hierarchy of genes responsible for lens regeneration in that it was Six-3 in conjunction with RA and not Pax-6 together with RA that induced lens regeneration.
Steroid Hormone Signaling - Retinoids
Retinoids and their receptors play major roles in both lens development and regeneration. Retinoids have been shown to affect morphogenesis and differentiation of several tissue types including the eye and limb. In the limb, retinoic acid has a proximalizing effect on limb regeneration due to its graded distribution and ability to affect the expression of Hox genes [52-54]. In the eye, exogenous retinoic acid has led to the formation of ectopic lens differentiation [55]. Following removal of the lens, treatment of newts with an antagonist to retinoic acid receptors or with disulfiram, which inhibits RA synthesis, severely retards the regenerative capability of the dorsal iris. While inhibition of lens regeneration was the most prevalent outcome, there were also a few cases of ectopic lenses [56,57]. As mentioned above, RA is essential along with Six-3 for inducing lens regeneration from the ventral iris [32]. The need for RA suggests that a synergism exists between RA and Six-3, which allows for the appropriate events to take place in order to induce lens regeneration. To date it is not known which factors RA regulates and how they function with Six-3 to promote lens regeneration.
Links between Regeneration and Development
In most vertebrates, lens development is initiated by proliferation of ectodermal cells overlying the optic vesicle forming the lens placode. The lens placode and the optic vesicle invaginate to form the lens pit and optic cup, respectively. The lens pit deepens and closes off forming the lens vesicle, which will ultimately form the lens. In the process of lens regeneration, events of differentiation are initiated after the formation of the lens vesicle. From this point on events of lens regeneration and development are very similar in terms of differentiation.
All of the factors and pathways known to be involved in lens regeneration were first shown to play a role in development. Throughout the years a plethora of genetic and molecular techniques have been used to elucidate the function of many of the key signaling factors in lens development. The research on lens development has significantly aided studies examining similar factors during regeneration. As hinted to in this review there are many differences and similarities between these two processes (Figure 2).
Figure 2.
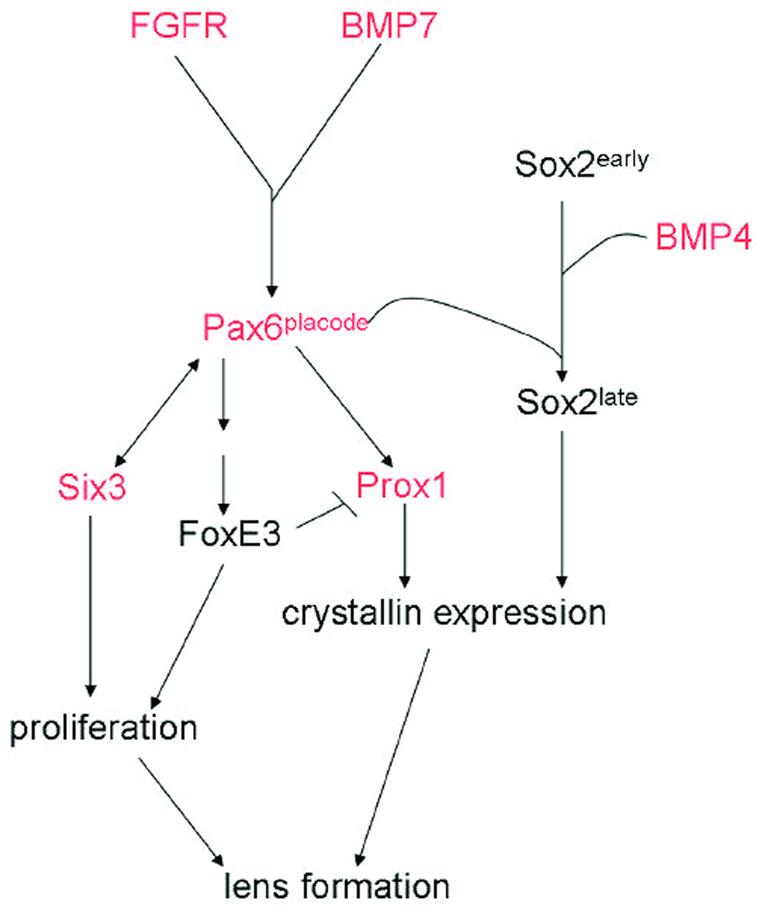
A diagram showing several factors involved in lens development. Some of these factors have also been examined in lens regeneration and are depicted with red color in the pathway. This might suggest conservation of function even though the inductive signals might be different.
Summary
Overall, this synthesis provides insight into the importance of signaling events during both lens development and lens regeneration. The study of signal transduction during lens development has provided hints into possible mechanisms underlying lens regeneration. However, care should be taken when utilizing this information, as it is clear that while there are many similarities between regeneration and development there also exist many significant differences. It is also apparent from this review that many signaling pathways may participate during lens regeneration, but the overall mechanism responsible for inducing regeneration remains elusive. It will take a concentrated effort to elucidate the complexity and cross talk responsible for such a remarkable phenomenon.
Acknowledgements
This work was supported in part by a National Eye Institute (NEI) grant EY10540 to P.A.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eguchi G. Electron microscopic studies on lens regeneration: I, mechanisms of depigmentatioon of the iris. Embryologia. 1963;8:45–62. [Google Scholar]
- 2.Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi G. Electron microscopic studies on lens regeneration: II, formation and growth of lens vesicle and differentiation of lens fibers. Embryologia. 1964;8:247–287. [Google Scholar]
- 4.Yamada T. Control mechanisms in cell-type conversion in newt lens regeneration. Krager; Basel: 1977. [PubMed] [Google Scholar]
- 5.Walsh PN, Schmaier AH. Platelet-coagulant protein interactions. In: Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 3rd ed. J.B. Lippincott Co.; Philadelphia: 1994. pp. 629–651. [Google Scholar]
- 6.Tanaka EM, Drechsel DN, Brockes JP. Thrombin regulates S-phase re-entry by cultured newt myotubes. Curr Biol. 1999;9:752–9. doi: 10.1016/s0960-9822(99)80362-5. [DOI] [PubMed] [Google Scholar]
- 7.Simon A, Brockes JP. Thrombin activation of S-phase reentry by cultured pigmented epithelial cells of adult newt iris. Exp Cell Res. 2002;281:101–6. doi: 10.1006/excr.2002.5650. [DOI] [PubMed] [Google Scholar]
- 8.Imokawa Y, Brockes JP. Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Curr Biol. 2003;13:877–81. doi: 10.1016/s0960-9822(03)00294-x. [DOI] [PubMed] [Google Scholar]
- 9.Imokawa Y, Simon A, Brockes JP. A critical for thrombin in vertebrate lens regeneration. Philos Trans R Soc Lond B Biol Sci. 2004;359:765–76. doi: 10.1098/rstb.2004.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Iongh RU, Lovicu FJ, Chamberlain CG, McAvoy JW. Differential expression of fibroblast growth factor receptors during rat lens morphogenesis and growth. Invest Ophthalmol Vis Sci. 1997;38:1688–99. [PubMed] [Google Scholar]
- 11.Lang RA. Which factors stimulate lens fiber cell differentiation in vivo? Invest Ophthalmol Vis Sci. 1999;40:3075–78. [PubMed] [Google Scholar]
- 12.Vogel-Höpker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000;94:25–36. doi: 10.1016/s0925-4773(00)00320-8. [DOI] [PubMed] [Google Scholar]
- 13.Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- 14.Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, Overbeek PA, Chepelinsky AB. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/Int-2 in transgenic mice. Dev Biol. 1998;198:13–31. doi: 10.1006/dbio.1998.8879. [DOI] [PubMed] [Google Scholar]
- 15.Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–77. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- 16.Del Rio-Tsonis K, Jung JC, Chiu IM, Tsonis PA. Conservation of fibroblast growth factor function in lens regeneration. Proc Natl Acad Sci USA. 1997;92:5092–96. doi: 10.1073/pnas.94.25.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–14. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Mizuno N, Owaribe K, Kuroiwa A, Okamoto M. Regulated lens regeneration from isolated pigmented epithelial cells of newt iris in culture in response to FGF2/4. Differentiation. 2002;70:101–108. doi: 10.1046/j.1432-0436.2002.700205.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Mizuno N, Ueda Y, Okamodo M, Kondoh H. FGF2 triggers iris-derived lens regeneration in newt eye. Mech Dev. 2004;121:519–26. doi: 10.1016/j.mod.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Yang EV, Wang L, Tassava RA. Effects of exogenous FGF-1 treatment on regeneration of the lens and the neural retina in the newt, Notophthalmus viridescens. J Exp Zoolog A Comp Exp Biol. 2005;303:837–44. doi: 10.1002/jez.a.215. [DOI] [PubMed] [Google Scholar]
- 21.Freeman G. Lens regeneration from the cornea of Xenopus laevis. J Exp Zool. 1963;154:39–66. doi: 10.1002/jez.1401540105. [DOI] [PubMed] [Google Scholar]
- 22.Bosco L, Venturini G, Willems D. In vitro lens transdifferentiation of Xenopus laevis outer cornea induced by Fibroblast Growth Factor (FGF) Development. 1997;124:421–8. doi: 10.1242/dev.124.2.421. [DOI] [PubMed] [Google Scholar]
- 23.Del Rio-Tsonis K, Trombley MT, McMahon G, Tsonis PA. Regulation of lens regeneration by fibroblast growth factor receptor 1. Dev Dyn. 1998;213:140–6. doi: 10.1002/(SICI)1097-0177(199809)213:1<140::AID-AJA14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt DS, Brahma SK, Courtois Y, Jeanny JC. Fibroblast growth factor receptors and regeneration of the eye lens. Dev Dyn. 1997;208:220–226. doi: 10.1002/(SICI)1097-0177(199702)208:2<220::AID-AJA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 26.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 27.Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–62. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–75. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 Acts in Murine Lens Placode Development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- 30.Dimanlig PV, Faber SC, Auerbach W, Makarenkova HP, Lang RA. The upstream ectoderm enhancer in Pax6 has an important role in lens induction. Development. 2001;128:4415–24. doi: 10.1242/dev.128.22.4415. [DOI] [PubMed] [Google Scholar]
- 31.Lang RA. Pathways regulating lens induction in the mouse. Int. J. Dev. Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- 32.Grogg MW, Call MK, Okamoto M, Vergara MN, Del Rio-Tsonis K, Tsonis PA. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature. 2005;438:858–62. doi: 10.1038/nature04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Iongh RU, Abud HE, Hime GR. WNT/frizzled signaling in eye development and disease. Front Biosci. 2006;11:2442–2464. doi: 10.2741/1982. [DOI] [PubMed] [Google Scholar]
- 34.Lyu J, Joo CK. Wnt signaling enhances FGF-2 triggered lens fiber cell differentiation. Development. 2003;131:1813–24. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- 35.Stump RJW, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/β-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 36.Smith AN, Miller LAD, Song N, Taketo MM, Lang RA. The duality of β-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–295. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from tyrian purple indirubins. Chem Biol. 2003;10:1255–66. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Lepourcelet M, Chen YNP, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 41.Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, Van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 42.Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- 43.Halder G, Callaerts P, Gehring WJ. New perspectives on eye evolution. Curr Opin Genet Dev. 1995;5:602–9. doi: 10.1016/0959-437x(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 44.Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- 45.Del Rio-Tsonis K, Washabaugh CH, Tsonis PA. Expression of Pax-6 during urodele eye development and lens regeneration. Proc Natl Acad Sci USA. 1995;92:5092–96. doi: 10.1073/pnas.92.11.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madhavan M, Haynes TL, Frisch NC, Call MK, Minich CM, Tsonis PA, Del Rio-Tsonis K. The role of pax-6 in lens regeneration. Proc Natl Acad Sci USA. 2006;103:14848–53. doi: 10.1073/pnas.0601949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–55. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- 48.Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Ectopic lens induction in fish in response to the murine homeobox gene Six3. Mech Dev. 1996;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- 49.Carl M, Loosli F, Wittbrodt J. Six3 inactivation reveals its essential role for the formation and patterning of the vertebrate eye. Development. 2002;129:4057–63. doi: 10.1242/dev.129.17.4057. [DOI] [PubMed] [Google Scholar]
- 50.Goudreau G, Petrou P, Reneker LW, Graw J, Loster J, Gruss P. Mutually regulated expression of Pax6 and Six3 and its implications for the Pax6 haploinsufficient lens phenotype. Proc Acad Natl Sci USA. 2002;99:8719–24. doi: 10.1073/pnas.132195699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–67. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- 52.Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982;295:672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- 53.Tsonis PA, Wahabaugh CH, Del Rio-Tsonis K. Morphogenetic effects of 9-cis-retinoic acid on the regenerating limbs of the axolotl. Roux's Arch. Dev. Biol. 1994;203:230–234. doi: 10.1007/BF00636339. [DOI] [PubMed] [Google Scholar]
- 54.Lu HC, Revelli JP, Goering L, Thalher C, Eichele G. Retinoid signaling is required for the establishment of a ZPA and for the expression of HoxB-8, a mediator of ZPA formation. Development. 1997;124:1643–1651. doi: 10.1242/dev.124.9.1643. [DOI] [PubMed] [Google Scholar]
- 55.Manns M, Fritzsch B. The eye in the brain: retinoic acid effects morphogenesis of the eye and pathway selection of axons but not the differentiation of the retina in Xenopus laevis. Neurosci Lett. 1991;127:150–154. doi: 10.1016/0304-3940(91)90782-o. [DOI] [PubMed] [Google Scholar]
- 56.Tsonis PA, Trombley MT, Rowland T, Chandraratna RA, Del Rio-Tsonis K. Role of retinoic acid in lens regeneration. Dev Dyn. 2000;219:588–93. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1082>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 57.Tsonis PA, Tsavaris M, Call MK, Chandraratna RAS, Del Rio-Tsonis K. Expression and role of retinoic acid receptor alpha in lens regeneration. Dev Growth Diff. 2002;44:391–394. doi: 10.1046/j.1440-169x.2002.00652.x. [DOI] [PubMed] [Google Scholar]