Abstract
It has been suggested that the degradation of the articular cartilage and osteoarthritis (OA) are associated with gender and the estrogen hormone. Although many investigators have reported the presence of the estrogen receptors (ERs) α and β in the articular cartilage, the localization of these receptors and the difference in their in vivo expression have not yet been clearly demonstrated. We performed immunofluorescence staining of ERα and ERβ to elucidate the localization of the ERs and to note the effects of gender and the aging process on these receptors. The results revealed that ERα and ERβ were expressed in the articular cartilage and subchondral bone layers of adult rats of both sexes. We also observed the high expression of these receptors in immature rats. In contrast, their expression levels decreased in an ovariectomised model, as a simulation of postmenopause, and in aged female rats. Therefore, this study suggests the direct effects of estrogen and ER expression on articular surface metabolism.
Keywords: estrogen hormone, estrogen receptor, articular cartilage, osteoarthritis, aging
I. Introduction
Primary osteoarthritis (OA) is a disease caused by the degradation of the articular cartilage. It occurs in a larger number of females than males, and the rates of both prevalence and incidence increase in postmenopausal women. Usually, the symptoms of OA are more severe in women than in men. Therefore, it has been suggested that both gender and the hormone estrogen may play a role in OA.
Estrogen has been known to act via estrogen receptors (ERs). Recently, it has also been reported that there are two subtypes of ERs, ERα and ERβ [7, 13]. Kuiper et al. demonstrated the distribution of both ERα and ERβ genes in rat tissues by using reverse transcription-polymerase chain reaction (RT-PCR). The expression of ERα was moderate to strong in the uterus, testes, pituitary, ovaries, kidneys, epididymis, and adrenal tissues. ERβ showed a similar expression pattern in the prostate, ovaries, lungs, bladder, brain, uterus, and testes [8]. Brandenberger et al. performed RT-PCR using human fetal tissues and found that ERα occurred most abundantly in the uterus, while high amounts of ERβ were found in the ovaries, testes, adrenal tissue, and spleen [2].
Ushiyama et al. demonstrated the expression of the ERα and ERβ genes in human articular chondrocytes by using RT-PCR. They reported that the expression of both the ERs was higher in men than in women. However, there was no difference between the joint sites or between the normal and osteoarthritic cartilage in this regard [24]. Although they showed that both the ERs are expressed in the articular cartilage, the localization of these receptors was not clearly demonstrated. To evaluate the localization of the ERs, Yamada et al. demonstrated using immunohistochemistry the staining of ERα in the articular cartilage of temporomandibular joint in the rat model [27]. ERα-positive chondrocytes were found more abundantly in the mature and hypertrophic cell layers of the condyle than in the fibrous and proliferative layers. Using immunohistochemistry, Nilsson et al. demonstrated the staining of ERβ in the human growth plate cartilage [14]. Chondrocytes in the hypertrophic layer were well stained, but no staining was observed in the chondrocytes in the resting and proliferative layers.
It has not been determined whether ERα and ERβ are expressed in all the layers of the loading joint articular cartilage. Further, the difference in the expression of ERα and ERβ and whether ER expression is affected by the aging process in vivo remain unclear.
In this study, we performed the immunofluorescence staining of ERα and ERβ in the rat femoral cartilage, paying special attention to the location of the ERs; we also attempted to identify differences arising due to the effects of sex, aging, or ovariectomy.
II. Materials and Methods
Animals
Male 3-month-old (n=3), and female 1-day (n=3), 1-week (n=3), 1-month (n=3), 3-month (n=6), and 24-month-old (n=3) Wistar rats were used. Moorthy et al. reported that young female rats (3 months old) show 4 or 5 days estrous cycles, but that during the aging process irregular cycles occur in some of the female rats at the age of 12 months [12]. Lu et al. reported that incidence of irregular cycle in aging rats increased to 65% over 12 months of age [10]. In this study 24-month-old rats were used as aging rats.
All experimental procedures were approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine, Japan.
Ovariectomy treatment
Female 3-month-old rats (n=3) were anesthetized by intraperitoneal injection of sodium pentobarbital (35 mg/kg body weight), and were bilaterally ovariectomized (OVX) under sterile surgical technique. Rats were allowed to move freely after the operation in a temperature-controlled environment with a 12-hr light-dark cycle, then sacrificed 1 month after OVX as previously described [10]. In this experiment we used OVX rats as a model in which the production of estrogen had decreased. The mean plasma concentration of estradiol in OVX-rats and 3-month-old female rats was 10 pg/ml and 40 pg/ml (30–50 pg/ml) (preliminary data), respectively.
Histological evaluation
Rats were anesthetized with sodium pentobarbital, perfused with physiological saline, then fixed with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). Distal parts of the femora were post-fixed in a fixative solution (4% PFA in 0.1 M PB) for 24 hr at 4°C, then decalcified with 0.5 M EDTA (pH 7.5, Wako, Osaka, Japan) for 2 weeks at room temperature (RT). After 2 weeks of decalcification, gradient replacement with 20% sucrose for 24 hr at 4°C was performed. The femora were then quick-frozen and cut into 14 µm thick sections on a cryostat (CM3050 S, Leica, Nussloch, Germany) as previously described [17, 18]. Finally, the sections were stained with 0.05% toluidine blue for histological examination.
Immunofluorescence staining
Subsequent sections, immediately following those which were stained with toluidine blue, were used for the immunofluorescence staining. The slides were rinsed 3 times with phosphate buffered saline 0.09% NaCl in 0.02 M PB for 5 min, then treated with 0.3% Triton X-100, 1% bovine serum albumin (BSA) in PBS for 30 min at RT. The slides were incubated for 60 hr with a rabbit polyclonal anti-ERα IgG (MC-20, Santa Cruz Biotechnology Inc., CA, USA) [22], or ERβ IgG (Z8P, Zymed Laboratories Inc., CA, USA) [3, 6, 16], at a dilution of 1:200 in 0.3% Triton X-100, 1% BSA in PBS at 4°C. The slides were rinsed 3 times with PBS for 5 min, then incubated with Alexa 488 labeled anti-rabbit IgG antibody at a dilution of 1:1000 in 0.1% Triton X-100, 2% BSA in PBS, for 2 hr at 4°C. Following a rinse (3 times) with PBS for 5 min at RT, the sections were embedded in Gelvatol (13% polyvinyl alcohol, 33% glycerol in 0.2 M Tris HCl) and were evaluated by confocal laser scanning microscope (LSM 510 META, ZEISS, Jena, Germany).
III. Results
Both ERα and ERβ antibodies are commercially available, and the specificity has been proved in many tissues [3, 6, 16, 22]. A positive control study showed the immunoreactivity for ERα and ERβ in PFA fixed pituitary tissue was located in the region of anterior lobe cells, which was the same reactivity as previously reported [15] (data not shown). In addition, a negative control study of immunohistochemical staining was performed using the same staining procedure, this time without the rabbit anti-mouse ERs. There were no signals in these particular sections (data not shown).
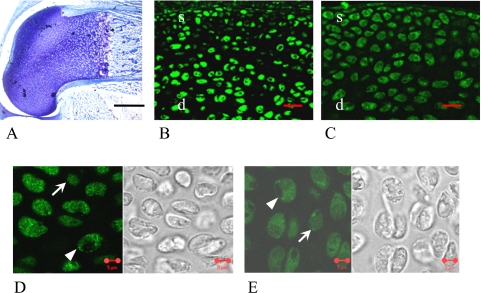
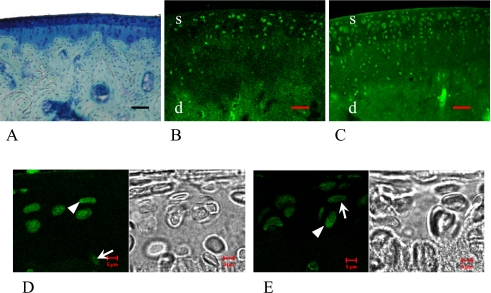
One-day-old female
The shape of femoral bone had already been formed, but the endochondral ossification was not complete at this time interval. Almost all epiphyseal areas showed metachromatic staining by toluidine blue, indicating the existence of cartilage matrix, and the cellularity of epiphyseal areas was very high (Fig. 1A). Almost all of the cells in both the superficial and the deep layers of epiphyseal areas were stained with ERα and ERβ including their nucleus and cytoplasm (Fig. 1D, E arrows, arrowheads). Both the cytoplasm and nuclei seemed to be stained in most cells; however, only the cytoplasm or only the nuclei were stained in some cells by these antibodies. There were no remarkable differences between the stained cell number of ERα and ERβ, and ERα tended to stain stronger than ERβ (Fig. 1B, C, Table 1).
Fig. 1.
1-day-old female rat. Femoral epiphyseal areas were stained by toluidine blue (A), indicating the existence of cartilage matrix. In the low magnification images, almost all of the cells in both the superficial (s) and the deep (d) layers of epiphyseal areas were stained with ERα (B) and ERβ (C). In the high magnification images, both nucleus and cytoplasm were stained diffusely with ERα (D) and ERβ (E). Arrowheads show the cells that were stained with ERs mainly in the cytoplasm, and arrows show the cells that were stained with ERs mainly in the nucleus. Bars=5 µm (A, D, E), 20 µm (B, C).
Table 1.
Changes of intracellular localization of ERs and immunoreactive level of ERs during aging process and by ovariectomy
| immunoreactive level of ERα | immunoreactive level of ERβ | |||
|---|---|---|---|---|
| cytoplasm nucleus | cytoplasm nucleus | |||
| one-day-old female | + | + | + | + |
| one-week-old female | + | + | + | + |
| one-month-old female | + | + | + | + |
| three-month-old female | + | + | + | + |
| three-month-old male | + | + | + | + |
| twenty four-month-old female | ± | ± | + | + |
| one month after OVX female | ± | ± | ± | ± |
Both ERα and ERβ were stained diffusely in not only nucleus but also cytoplasm. With aging, the immunoreactivity level of ERα and ERβ got weaker, and the aged female rats and OVX rats exhibited lower number of stained cells than immature and mature female rats. (+); higher immunoreactivity, (±); lower immunoreactivity.
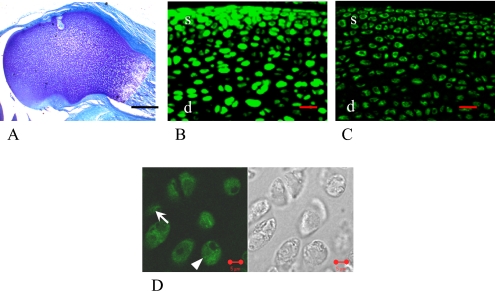
One-week-old female
Almost all of the epiphyseal areas still showed metachromatic staining by toluidine blue at this time interval (Fig. 2A). Nearly all cells in both the superficial and the deep layers were stained with ERα and ERβ including their nucleus and cytoplasm (Fig. 2D arrow, arrowhead). There was no remarkable change compared to the one-day-old models, and the cells still stained stronger by ERα than by ERβ (Fig. 2B, C, Table 1).
Fig. 2.
1-week-old female rat. In the low magnification images, femoral epiphysial areas still stained by toluidine blue (A), in which almost all of the cells in both the superficial (s) and the deep (d) layers were stained with ERα (B) and ERβ (C). In the high magnification image, arrowhead shows the cell that was stained with ERs mainly in the cytoplasm, and arrow shows the cell that was stained with ERs mainly in the nucleus (D). Bars=5 µm (A, D), 20 µm (B, C).
One-month-old female
Endochondral ossification was started, and subchondral bone had formed; however, articular cartilage was not well developed at this time interval (Fig. 3A). Almost all of the cells in both the articular cartilage and subchondral bone layers were stained with ERα and ERβ. The strength of the ERα signal was similar to that of ERβ (Fig. 3B, C, Table 1).
Fig. 3.
1-month-old female rat. Articular surface was constructed with articular cartilage and subchondral bone layers (A). Almost all of the cells in both the articular cartilage and subchondral bone layers were stained with ERα (B) and ERβ (C). Bar=50 µm.
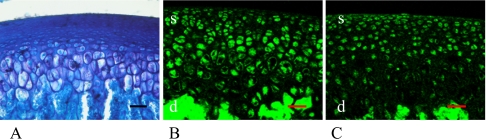
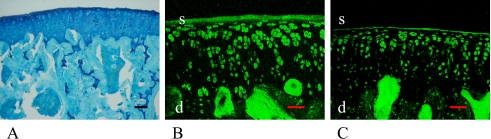
Three-month-old female and male
Articular cartilage and subchondral bone were mature and well formed at this time interval (Figs. 4A, 5A). In the female rats, almost all of the cells in the superficial layer of the articular cartilage were stained with ERα and ERβ (Fig. 4B, C). However, some of the cells in the deeper layers of articular cartilage and subchondral bone layers were not stained with both ERs (Fig. 4B, C arrows). Similarly, almost all of the male rat cells expressed both ERα and ERβ. This was particularly evident in the superficial layer (Fig. 5B, C, Table 1).
Fig. 4.
3-month-old female rat. Cartilage and bone formations were mature (A). In female, almost all of the cells in the superficial (s) layer (arrows) of the articular cartilage were stained with ERα (B) and ERβ (C). Bar=50 µm.
Fig. 5.
3-month-old male rat. Similar to female, almost all the cells, especially in the superficial (s) layer of articular cartilage (A) expressed both ERα (B) and ERβ (C). Bar=50 µm.
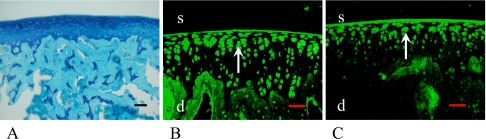
Twenty-four-month-old female
There was no remarkable degradative change on the articular surface; however, the thickness of cartilage layer was thinner than that of the younger models (Fig. 6A). Some of the cells in the superficial layer of the articular cartilage were stained with ERα and ERβ including nucleus and cytoplasm (Fig. 6D, E arrows, arrowheads), but the number of ERα stained cells had decreased. Only a few cells in the deeper layers of articular cartilage and subchondral bone were stained with both ERα and ERβ (Fig. 6B, C, Table 1).
Fig. 6.
24-month-old female rat. There was no remarkable degenerative change on the articular surface; however, the cartilage thickness was thinner than that of the younger models (A). In the low magnification images, some cells in the superficial (s) and a few cells in the deeper (d) layer of the articular cartilage were stained with ERα (B) and ERβ (C). In the high magnification images, both nucleus and cytoplasm were stained diffusely with ERα (D) and ERβ (E). Arrowheads show the cells that were stained with ERs mainly in the cytoplasm, and arrows show the cells that were stained with ERs mainly in the nucleus. Bars=50 µm (A–C), 5 µm (D, E).
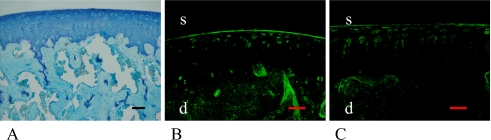
One month after OVX female
There were no remarkable symptoms of inflammation or infection after OVX treatment. Compared to the articular surface of 3-month-old female, there was no remarkable degradation or osteoarthritic change 1 month after OVX in toluidine blue staining (Fig. 7A). While there was no difference between ERα and ERβ, the number of stained cells had dramatically decreased, and the results were similar to those of aged rat models (Fig. 7B, C, Table 1).
Fig. 7.
4-month-old female rat 1 month after OVX. There was no remarkable degradational or osteoarthritic change (A). Similar to the results of aged rat models, some cells were stained with ERα (B) and ERβ (C). Bar=50 µm.
IV. Discussion
In order to evaluate the localization and changes of ERα and ERβ expressions in articular surface due to gender, aging, or ovariectomy, immunofluorescence staining was performed using a rat model. The results showed that both ERα and ERβ were located and expressed in the articular cartilage and subchondral bone of young and mature female rats and mature male rats. In young rats, the degree of ERα staining appeared to be stronger than that of ERβ; however, the number of stained cells was similar between ERα and ERβ. With aging or by OVX, the degree of staining of the ERα became weaker. As compared to the mature female rats, the aged female rats and OVX rats exhibited a marked lower number of ERα stained cells. In aging rats, the staining degree of ERβ was same as in mature female rats. The function of ERβ may be different from that of ERα.
Monje and Boland reported that ERα and ERβ were localized not only to the nucleus but also the cytoplasm within the cells in rabbit uterus and ovary [11]. Levin demonstrated that signal transduction had occurred through ERs in the nucleus, plasma membrane, and cytoplasm including mitochondria [9]. In our study, ERs were localized in both nucleus and cytoplasm in the cartilage tissue. As to the significance of the intracellular localization of ERα and ERβ, it is possible that signal pathways through nucleus and cytoplasm including mitochondria depend on the estrogen hormone. Estrogen may have some direct effects on the chondrocytes through the estrogen receptor.
Because the prevalence and incidence of OA differs not only between the genders but also between premenopausal and postmenopausal women, it is considered that degradation of the articular cartilage is associated with the estrogen hormone, and that estrogen replacement therapy (ERT) has an effect on the articular cartilage. Ravn et al. used oral and transdermal estradiol therapies and evaluated cartilage degradation and bone resorption by measuring the content of C-telopeptides of type I and type II collagen in urine by using an enzyme-linked immunosorbent assay (ELISA) [19]. Using magnetic resonance imaging, Wluka et al. calculated the difference in the amount of knee cartilage in women over 50 years who either had or had not undergone ERT [25]. In their human study, they concluded that in postmenopausal women, ERT provided protected against OA and osteoporosis. However, later in their longer follow-up study, Wluka et al. later reported that ERT had no effect on the cartilage [26]. Further, Tsai and Liu reported that an intraarticular injection of estradiol actually induced knee OA in their rabbit study. In their experiment, the change in OA was clearly different between the high- and low-dose groups as well as between the short and long periods [23]. Further, some other investigators have also reported that ERT has no significant effect on the articular cartilage. They considered that conflicting results were obtained because of the differences between human and animal species, sample sizes, in vivo and in vitro models, estradiol dosage, and duration of the treatment [23, 24]. Thus, the effect of estrogen on cartilage has not yet been determined [1, 4, 20].
It has been recognized that there is a relationship between estrogen and bones, and that estrogen halts or reduces bone loss in women [1]. Estrogen deficiency causes osteoporosis, and, therefore, the decreasing mechanical strength of the subchondral bones may indirectly affect the articular cartilage [25]. It is also well known that the serum estrogen level in males is markedly lower and does not change significantly throughout their life span. Conversely, the serum estrogen level in women is considerably higher and decreases significantly after menopause. Our results showed that ERα and ERβ were expressed on the articular surface in mature males and both immature and mature females. Because the plasma estradiol level did not differ between young male (5 months old) and old male (24 months old) rat [21], the difference of ER expression was not considered to be associated with the aging process. The levels of ERα and ERβ in the articular cartilage of the OVX rats, as a simulation of postmenopause, as well as the levels of ERα in aged female rats were found to be reduced. These results suggest that estrogen and ERα expression directly affect cartilage metabolism throughout life, irrespective of whether or not this effect is beneficial. We demonstrated the presence of ERs in the articular cartilage of the loading joint by using immunofluorescence staining. These results provide basic data that will facilitate further research on the relationship among OA, aging, sex, estrogen, and ERs.
V. Acknowledgments
This work was supported in part by Grants-in-Aid from Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology, Japan (K. M. and M. K.).
VI. References
- 1.Barrett-Connor E., Stuenkel C. A. Hormone replacement therapy (HRT)—risks and benefits. Int. J. Epidemiol. 2001;30:423–426. doi: 10.1093/ije/30.3.423. [DOI] [PubMed] [Google Scholar]
- 2.Brandenberger A. W., Tee M. K., Lee J. Y., Chao V., Jaffe R. B. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J. Clin. Endocrinol. Metab. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 3.Creutz L. M., Kritzer M. F. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J. Comp. Neurol. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- 4.Felson D. T., Nevitt M. C. Estrogen and osteoarthritis: how do we explain conflicting study results? Prev. Med. 1999;28:445–448. 449–450. doi: 10.1006/pmed.1999.0491. discussion . [DOI] [PubMed] [Google Scholar]
- 5.Hoegh-Andersen P., Tanko L. B., Andersen T. L., Lundberg C. V., Mo J. A., Heegaard A. M., Delaisse J. M., Christgau S. Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res. Ther. 2004;6:R169–180. doi: 10.1186/ar1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hrabovszky E., Steinhauser A., Barabas K., Shughrue P. J., Petersen S. L, Merchenthaler I., Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- 7.Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuiper G. G., Carlsson B., Grandien K., Enmark E., Haggblad J., Nilsson S., Gustafsson J. A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 9.Levin E. R. Integration of the extranuclear and nuclear actions of estrogen. Mol. Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu K. H., Hopper B. R., Vargo T. M., Yen S. S. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol. Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 11.Monje P., Boland R. Subcellular distribution of native estrogen receptor alpha and beta isoforms in rabbit uterus and ovary. J. Cell. Biochem. 2001;82:467–479. doi: 10.1002/jcb.1182. [DOI] [PubMed] [Google Scholar]
- 12.Moorthy K., Sharma D., Basir S. F., Baquer N. Z. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp. Gerontol. 2005;40:295–302. doi: 10.1016/j.exger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Mosselman S., Polman J., Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson L. O., Boman A., Savendahl L., Grigelioniene G., Ohlsson C., Ritzen E. M., Wroblewski J. Demonstration of estrogen receptor-beta immunoreactivity in human growth plate cartilage. J. Clin. Endocrinol. Metab. 1999;84:370–373. doi: 10.1210/jcem.84.1.5531. [DOI] [PubMed] [Google Scholar]
- 15.Nishihara E., Nagayama Y., Inoue S., Hiroi H., Muramatsu M., Yamashita S., Koji T. Ontogenetic changes in the expression of estrogen receptor alpha and beta in rat pituitary gland detected by immunohistochemistry. Endocrinology. 2000;141:615–620. doi: 10.1210/endo.141.2.7330. [DOI] [PubMed] [Google Scholar]
- 16.Orikasa C., Kondo Y., Hayashi S., McEwen B. S., Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc. Natl. Acad. Sci. U S A. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshima Y., Watanabe N., Matsuda K., Takai S., Kawata M., Kubo T. Fate of transplanted bone-marrow-derived mesenchymal cells during osteochondral repair using transgenic rats to simulate autologous transplantation. Osteoarthritis Cartilage. 2004;12:811–817. doi: 10.1016/j.joca.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Oshima Y., Watanabe N., Matsuda K., Takai S., Kawata M., Kubo T. Behavior of transplanted bone marrow-derived GFP mesenchymal cells in osteochondral defect as a simulation of autologous transplantation. J. Histochem. Cytochem. 2005;53:207–216. doi: 10.1369/jhc.4A6280.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ravn P., Warming L., Christgau S., Christiansen C. The effect on cartilage of different forms of application of postmenopausal estrogen therapy: comparison of oral and transdermal therapy. Bone. 2004;35:1216–1221. doi: 10.1016/j.bone.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Reginster J. Y., Kvasz A., Bruyere O., Henrotin Y. Is there any rationale for prescribing hormone replacement therapy (HRT) to prevent or to treat osteoarthritis? Osteoarthritis Cartilage. 2003;11:87–91. doi: 10.1053/joca.2002.0872. [DOI] [PubMed] [Google Scholar]
- 21.Roselli C. E., Thornton J. E., Chambers K. C. Age-related deficits in brain estrogen receptors and sexual behavior of male rats. Behav. Neurosci. 1993;107:202–209. doi: 10.1037//0735-7044.107.1.202. [DOI] [PubMed] [Google Scholar]
- 22.Shim W. S., DiRenzo J., DeCaprio J. A., Santen R. J., Brown M., Jeng M. H. Segregation of steroid receptor coactivator-1 from steroid receptors in mammary epithelium. Proc. Natl. Acad. Sci. U S A. 1999;96:208–213. doi: 10.1073/pnas.96.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai C. L., Liu T. K. Estradiol-induced knee osteoarthrosis in ovariectomized rabbits. Clin. Orthop. Relat. Res. 1993;291:295–302. [PubMed] [Google Scholar]
- 24.Ushiyama T., Ueyama H., Inoue K., Ohkubo I., Hukuda S. Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage. 1999;7:560–566. doi: 10.1053/joca.1999.0260. [DOI] [PubMed] [Google Scholar]
- 25.Wluka A. E., Davis S. R., Bailey M., Stuckey S. L, Cicuttini F. M. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann. Rheum. Dis. 2001;60:332–336. doi: 10.1136/ard.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wluka A. E., Wolfe R., Davis S. R., Stuckey S., Cicuttini F. M. Tibial cartilage volume change in healthy postmenopausal women: a longitudinal study. Ann. Rheum. Dis. 2004;63:444–449. doi: 10.1136/ard.2003.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada K., Nozawa-Inoue K., Kawano Y., Kohno S., Amizuka N., Iwanaga T., Maeda T. Expression of estrogen receptor alpha (ER alpha) in the rat temporomandibular joint. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;274:934–941. doi: 10.1002/ar.a.10107. [DOI] [PubMed] [Google Scholar]