Abstract
The proper assembly of sperm flagellar proteins is fundamental for sperm motility. The sperm- and spermatid-specific isoform of glyceraldehyde 3-phosphate dehydrogenase, GAPDS, is a flagellar protein indispensable for sperm flagellar movement. To obtain information on the assembly of the glycolytic enzyme into the flagellum, the precise localization of rat GAPDS in the flagellum and the stage of incorporation into the flagellum were examined using a monoclonal antibody. Immunolocalization of rat GAPDS was restricted to the fibrous sheath (FS) in the sperm flagellum, and was predominant in the circumferential ribs rather than the longitudinal columns. Immunoreactivity was first detected in the cytoplasm and flagella of the step-16 spermatids during the final step of FS formation. Together with the expression of other FS proteins, the present results indicate the sequential assembly of FS components, suggesting that the expression and transport of GAPDS is regulated in a coordinated manner during sperm flagellar formation.
Keywords: fibrous sheath, flagellum, sperm, spermiogenesis, rat
I. Introduction
The proper assembly of the flagellar components is essential for effective flagellar movement of the sperm to swim up as far as the egg for fertilization. In fact, a number of cases of dysplasia of FS have been reported in asthenozoospermic sterile men [2]. In this respect, it is of interest to determine whether the synthesis, transport and incorporation into the flagellum of flagellar proteins are regulated in coordination with the construction of the flagellar cytoskeletal framework. The process of biogenesis of the flagellum has been carefully analyzed in the rat in an electron microscopic study on the morphogenesis of FS in rat spermatids that demonstrated the sequence of the assembly of the structure [6]. FS is composed of two longitudinal columns and numerous transverse ribs connecting the columns. The longitudinal columns develop during early spermiogenesis and the ribs subsequently emerge and develop during late spermiogenesis [6]. During the period of FS development, newly synthesized proteins are incorporated into the developing FS [6]. However, the expression and assembly of rat FS components during spermiogenesis have not been precisely examined except for A-kinase anchoring protein 4 (AKAP4) [15] and tissue-specific testicular thioredoxin-2 (SPTRX-2) [11].
Mouse and rat GAPDS and its human orthologue (GAPD2) are the sole isozyme of glyceraldehyde-3-phosphate dehydrogenase in the sperm [3, 20–22]. GAPDS is intimately associated with the fibrous sheath and provides most of the energy source for sperm motility [10]. In this context, the process of incorporation of GAPDS into FS during flagellar formation will be a valuable model of the assembly of non-structural proteins into FS. The objectives of the present study are to examine the precise localization of rat GAPDS and to determine its temporal sequence of expression during flagellar development. The results show that rat GAPDS is preferentially incorporated into the ribs of FS during the final stage of the FS formation.
II. Materials and Methods
Animals
Wistar rats and BALB/c mice were allowed free access to food and water. Mice were sacrificed by cervical dislocation and rats were sacrificed after being anesthetized with ether. All experiments were approved by the Animal Research Committee of University of Miyazaki.
Preparation of monoclonal antibody
The method of monoclonal antibody production was described previously [17]. In brief, testicular cells from 12 day-old rats were treated with non-ionic weak detergent, 40 mM n-heptyl-β-d-thioglucoside (Wako Pure Chemical Industries, Osaka, Japan) on ice for 10 min. The extract was centrifuged at 20,000 g for 30 min at 4°C and the resultant supernatant was used as an immunogen. Adult female Balb/c mice were immunized subcutaneously. Spleen cells were collected from the immunized mice and fused with NS-1 myeloma cells. The monoclonal antibody obtained is referred to as MC321 (IgM).
Ascites containing MC321 were obtained by intraperitoneal injection of antibody-producing hybridoma cells into Balb/c female mice. MC321 was purified from the ascites by Mono Q (Amersham Biosciences, Piscataway, NJ, USA) column chromatography. For immunohistochemistry, immunoelectron microscopy, and Western blotting, MC321 was used at a 1:80 dilution from the spent culture medium.
Antigen extraction and Western blotting
Testes from 12-day old and adult rats were dissected and suspended in 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM 2-mercaptoethanol, and protease inhibitors containing 1 mM PMSF (Sigma Chemical Co., St Louis, MO, USA), 5 µg/ml leupeptin (Peptide Institute, Osaka, Japan), 5 µg/ml pepstatin A (Peptide Institute), and 5 µg/ml aprotinin (Nacalai Tesque, Kyoto, Japan). To obtain the cytosol of testicular cells, the cells were disrupted by nitrogen decompression with Parr Cell Disruption Bomb (Parr Instrument Company, Moline, IL, USA). Pressure was maintained at 900 psig for 20 min on ice. Homogenates were centrifuged at 1,500 g for 10 min, followed at 20,000 g for 40 min. The resultant supernatants were mixed with equal volume of 2×SDS-sample buffer containing 0.25 M Tris-HCl (pH 6.8), 4% SDS, 10% 2-mercaptoethanol, and 10% sucrose. Sperm proteins were obtained from the rat cauda epididymides by extraction with SDS-sample buffer at 100°C for 3 min. After boiling for 3 min, samples were subjected to PAGE on 7.5% separating gels. The proteins were transferred onto polyvinylidiene fluoride (PVDF) membranes. The blots were then probed with MC321.
Busulfan treatment
Busulfan (10 mg/kg body weight), a cytotoxic agent that destroys most of the stem spermatogonia, was administered to adult rats by intraperitoneal injection as described previously [7]. Approximately 24 days after the injection of a single dose of the agent, spermatogenesis recovers. In the testes at 58 days after the busulfan injection, step-8 spermatids comprised the most differentiated population of spermatogenic cells and all later spermatids in steps 9–19 were absent. The rats were killed after the treatment and the testes were excised.
Partial purification and identification of the 64-kDa antigenic protein
An immunoaffinity column was prepared by cross-linking of MC321 with Affigel 10 (Bio-Rad Laboratories, Richmond, CA, USA) according to the manufacturer’s instructions. The testicular extracts were applied to the MC321-immunoaffinity column equilibrated with PBS. The column was thoroughly washed with 20 column volumes of PBS. Proteins specifically bound to the column were eluted with the elution buffer containing 50 mM glycine (pH 2.5), 0.15 M NaCl, 2 mM EDTA, and protease inhibitors.
To identify the antigenic protein for MC321, the proteins isolated by the MC321-immunoaffinity chromatography were resolved in an SDS-7.5% polyacrylamide gel and visualized by Coomassie brilliant blue staining. Portions of the gel containing an immunoreactive protein were excised, and then digested with trypsin as previously described [14]. Peptides eluted from gel pieces were analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) using a Voyager Elite system (Applied Biosystems, Tokyo, Japan). Identified peptides were compared with peptide sequences of annotated coding regions by applying the MASCOT search program.
Immunoelectron microscopy
Mature rat testes were fixed by perfusion of 4% paraformaldehyde+0.3% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.4) through the testicular artery and then immersed in the same fixative overnight at 4°C. The specimens were embedded in Lowicryl K4M. Ultrathin sections on nickel grids were immunogold-labeled for electron microscopy as described [18]. MC321 was used as a primary antibody, and 15-nm gold-conjugated antimouse IgM antibody (EY Laboratories Inc., San Mateo, CA) was used as a secondary antibody at a 1:200 dilution. Control experiments were carried out by replacing the primary antibody with normal mouse IgM or PBS. Counterstaining was done using uranyl acetate/methyl cellulose solution [16]. Sections were observed under a transmission electron microscope (Hitachi model H7100, Tokyo, Japan) at an accelerating voltage of 75 kV.
Immunohistocytochemistry
Rat tissues were fixed with Bouin’s fixative by perfusion through the thoracic aorta and embedded in paraffin. Immunoperoxidase staining was performed on deparaffinized sections as described [17]. MC321 was used as a primary antibody. Control experiments were carried out by replacing the primary antibody with normal mouse IgM or PBS. Some sections were counterstained with hematoxylin, or observed with Nomarski’s differential interference contrast without counterstaining. Periodic acid Schiff (PAS) stained consecutive sections were used for identification of the stages of the cycle of the seminiferous epithelium according to the classification of Hess [5].
Sperm were prepared from the rat cauda epididymis, washed twice with PBS by centrifugation at 250 g for 5 min, and attached to slide glasses by cytospin. The immunostaining procedure is described above.
III. Results
Western blot analysis of the antigens
We produced monoclonal antibodies by using extracts of immature rat testes on postnatal day 12 as an immunogen. As a result, we established three clones producing monoclonal antibody, including MC321 [17].
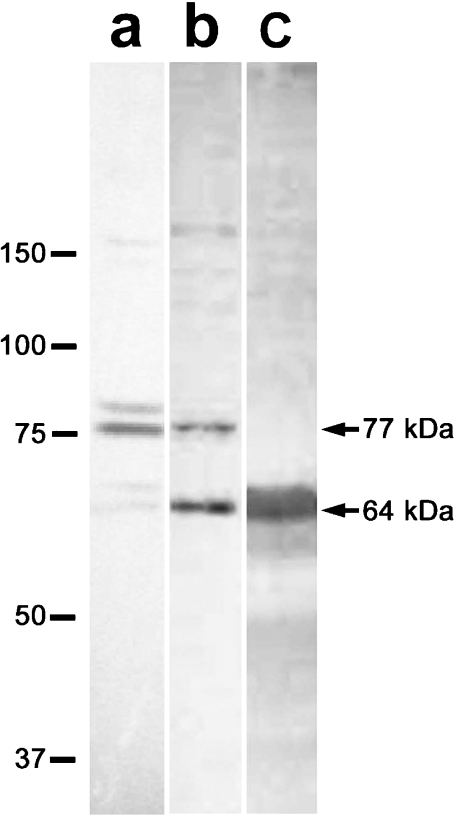
The molecular weight of the antigen was determined by Western blotting with MC321 (Fig. 1). In the testicular extracts from 12-day-old rats, an immunoreactive band of 77 kDa was prominent and another minor band was detected at a position above the 77-kDa band. The testicular cytosolic proteins prepared from adult rats contained two immunoreactive proteins with relative molecular masses of 77 kDa and 64 kDa. In the sperm proteins extracted with 2% SDS, the 64-kDa band was the only immunoreactive one.
Fig. 1.
Detection of the antigen on Western blot. Proteins were obtained from 12-day testes (a), adult testis (b) and epididymal sperm (c). Note that the 64-kDa antigenic protein is present in both adult testes and sperm, but not in the 12-day testes. Numbers on left indicate molecular weight markers (×10−3).
Different origins of the two antigens in the adult testis
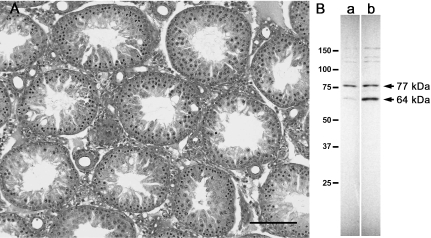
Two antigenic proteins of 64-kDa and 77-kDa were detected in the testicular extract from adult rats (Fig. 1b). To determine the origin of the antigens, we used the adult testes at 58 days after the injection of a single dose of busulfan, in which most of the elongating spermatids and testicular sperm were depleted by the treatment (Fig. 2A). In the Western blots of the extract from the busulfan-treated testes, the 77-kDa protein was prominent while the 64-kDa protein was hardly detected (Fig. 2B). This result shows that the 64-kDa protein is derived exclusively from the sperm and elongating spermatids. Together with the finding that the 77-kDa band was prominent in the 12-day testes, the 77-kDa protein is presumably derived from Sertoli cells, meiotic or premeiotic spermatogenic cells.
Fig. 2.
Immunodetection of the antigen in busulfan-treated adult testes. A: Section of busulfan-treated testis stained with hematoxylin-eosin. Bar=200 µm. B: Western blotting. Proteins were obtained from busulfan-treated testes (a) and control testes without treatment (b). Numbers on left indicate molecular weight markers (×10−3).
Partial purification and identification of the antigenic 64-kDa protein
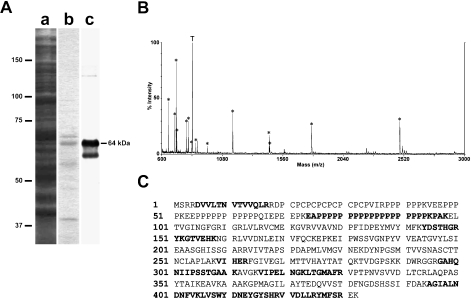
To isolate the antigens, testicular cytosolic proteins from adult rats were loaded onto an MC321-immunoaffinity column, and the proteins specifically bound to the column were then eluted and analyzed by SDS-PAGE and Western blotting. In the gel stained by Coomassie brilliant blue (CBB), the 64-kDa protein was predominant, while several minor proteins with relative molecular masses of 67 kDa and 39 kDa were detected in the eluate (Fig. 3A). Among them, the 64-kDa protein was immunoreactive while the other proteins showed no immunoreactivity. An immunoreactive band of 59 kDa was detected in the Western blots, but no corresponding band was found in the CBB stained gel. Neither the CBB stained band nor immunoreactive band for the 77-kDa protein was detectable in the eluate. To identify the 64-kDa protein, a portion of the 64-kDa gel band was excised and submitted for proteolytic digestion and peptide identification by MALDI-TOF MS. As a result, the 64-kDa protein was unambiguously identified as GAPDS (GenBank accession no. AJ297631) (Fig. 3B, C).
Fig. 3.
Partial purification and identification of the antigenic 64-kDa protein. A: Partial purification by MC321-immunoaffinity chromatography. Total proteins (a) or proteins isolated by MC321-immunoaffinity chromatography (b, c) were resolved in reducing SDS gels, and visualized with Coomassie brilliant blue (a, b), or examined by Western blotting with MC321 (c). Numbers on left indicate molecular weight markers (×10−3). B: MALDI-TOF mass spectrum of the 64-kDa protein after in-gel trypsin digestion. Asterisk labeled peptides matched the rat GAPDS sequence. A tryptic peptide is indicated by T. C: Rat GAPDS sequence. Peptides in bold have been matched to the peptides in MALDI MS.
Immunolocalization of GAPDS in the flagellum
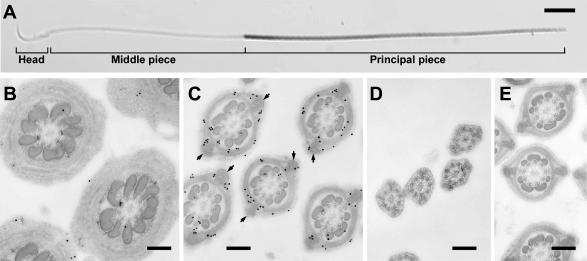
Western blot analysis indicated that the only antigenic molecule in rat sperm was GAPDS (Fig. 1). Immunocytochemistry demonstrated that GAPDS was restricted to the principal piece and was not found in other sperm domains including the head, midpiece and end piece of the rat epididymal sperm (Fig. 4A). The specific localization in the principal piece was further analyzed in late step-19 spermatids by post-embedding immunogold labeling for electron microscopy. Gold particles were preferentially labeled on FS which is a specific structure in the principal piece (Fig. 4B–D). No specific labeling was detectable on the outer dense fiber, axoneme and mitochondrial sheath. FS is composed of two distinct components: the longitudinal columns and circumferential ribs. Gold particles appeared to be predominant in the ribs rather than in the longitudinal columns (Fig. 4C). When we counted the number of gold particles in 200 transverse sections of the principal piece, the density of the gold particles within the whole area of the ribs was approximately 3.8 times greater than that within the area of the longitudinal column. Notably, the central zone of the longitudinal columns was scarcely labeled with gold particles (Fig. 4C). No gold labeling was shown on FS in the control experiments (Fig. 4E).
Fig. 4.
Immunolocalization of GAPDS. A: Immunocytochemical localization of GAPDS in epididymal sperm. Note the specific immunoreactivity in the principal piece. Bar=10 µm. B–E: Immunogold labeling for GAPDS on the midpiece (B), principal piece (C), and end piece (D) of the late step-19 spermatid flagella. Control on the section of the principal piece (E). Micrographs in B–E were obtained from the same section for comparison. Note the predominant specific immunogold labeling on the fibrous sheath, while the center of the longitudinal columns (arrows) is scarcely labeled. Bars=0.2 µm.
Expression of GAPDS during spermiogenesis
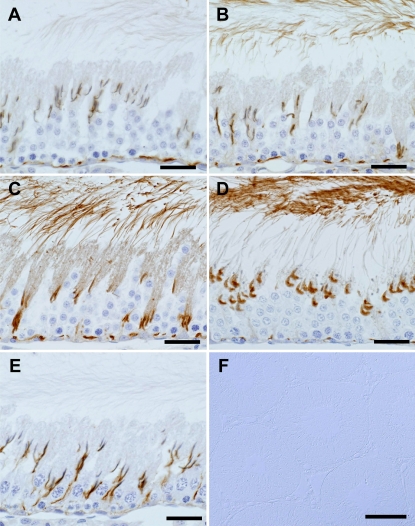
We next determined the distribution and change in expression of GAPDS in adult testes by immunohistochemistry. Immunoreactivity was detected in the cytoplasm and flagella of the elongating spermatids, as well as at the sites near the basal membrane and around the head caps of the elongating spermatids (Fig. 5A–E). Although multiple sites were immunoreactive, judging from the results of the experiment with the busulfan-treated testes (Fig. 2), the immunoreactivity in the cytoplasm and flagella of the elongating spermatids corresponds to GAPDS. The immunoreactivity around the head cap is assumed to be localized either to spermatid and/or Sertoli cells. To determine the precise localization, post-embedding immunogold staining was done in the testis. Gold particles were restricted to the actin layer of ectoplasmic specialization in Sertoli cells (Fig. 6). No or scarcely any gold labeling was found on the nucleus and acrosome of the elongating spermatids. Control experiments showed no distinct gold labeling. The immunoreactive sites near the basal membrane correspond to the blood-testis barrier which is formed by the inter-Sertoli tight junctions. In this study, we focused on the temporal sequence of the GAPDS expression appearing in the cytoplasm and flagellum of the elongating spermatids. In the seminiferous tubules at stage II, a weak immunoreaction was first detectable in both the cytoplasm and the flagella of the early step-16 spermatids (Fig. 5A). In the earlier stages, no immunoreaction was detectable in either the flagella or cytoplasm of the spermatids (Fig. 5E). The intensity of the immunoreaction increased as spermiogenesis proceeded (Fig. 5B, C), reached a maximum in the late step-17 spermatids in the stage-V seminiferous tubules (Fig. 5C), and persisted until the terminal stage of spermiogenesis (Fig. 5D). No immunoreactivity was found in the nucleus of any cells in the testis, nor in the cytoplasm of spermatogonia, spermatocytes, peritubular myoid cells, Leydig cells, or blood vessels. The control sections with the preimmune serum showed no immunoreactivity (Fig. 5F).
Fig. 5.
Analysis of precise stage of protein expression during the seminiferous cycle in adult testis. Seminiferous tubules in different stages were immunostained with MC321. A: Stage II. B: Stage III. C: Stage V. D: Stage VII. E: Stage XIII. The sections were counterstained with hematoxylin. Note that the immunoreactivity in the cytoplasm and flagella of the spermatids varies with the spermatogenic cycle stage. Bars=50 µm (A–E). F: Control section with the preimmune serum without counterstaining. DIC images. Bar=200 µm.
Fig. 6.
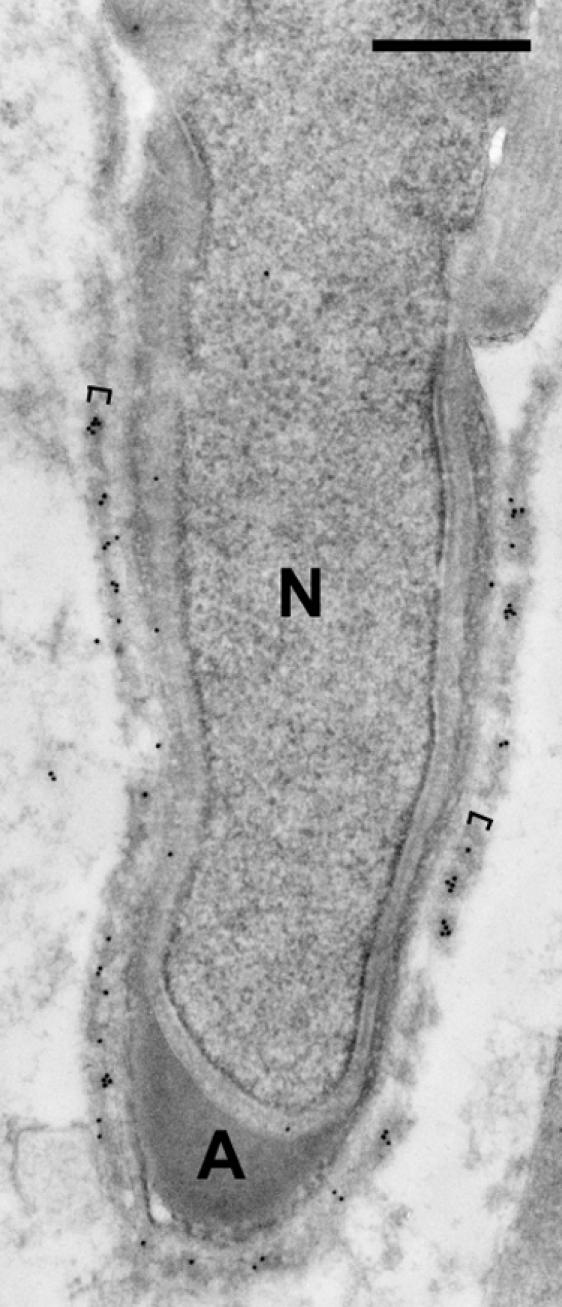
Immunogold staining of the Sertoli-elongating spermatid junction. Note the specific immunogold labeling of the actin layer (brackets) of ectoplasmic specialization in Sertoli cell surrounding the step-11 spermatid. N, nucleus; A, acrosome. Bar=0.5 µm.
IV. Discussion
Although the antibody used in this study recognizes two proteins of 64 kDa and 77 kDa in the adult rat testes, the origin of the 64-kDa protein was distinguishable from that of the 77-kDa protein by the experiment using the busulfan-treated rat testes, which showed that the 64-kDa protein is derived exclusively from sperm and spermatids. Mass spectrometry demonstrated that the 64-kDa protein is GAPDS. By these analyses, the antibody can be used as a tool for the precise localization and expression of rat GAPDS.
Our immunogold electron microscopic study showed a preferential labeling of rat GAPDS on the circumferential ribs rather than on the longitudinal column of FS. However, a previous study reported that mouse GAPDS distributes on both the columns and ribs [3]. The discrepancy is probably due to the methods used for localization of the enzymes. The pre-embedding immunoperoxidase staining method used in the previous study is not adequate for quantitative analysis. Similarly to rat GAPDS, AKAP3/FSP95 is predominantly localized to the ribs in human sperm and spermatids by post-embedding immunogold electron microscopy [9]. Ropporin and AKAP-associated sperm protein (ASP) have been identified as AKAP3 binding proteins by the yeast two hybrid system [1]. Both proteins share a strong sequence similarlity with the AKAP-binding domain of the regulatory (RIIα) subunit of A-kinase. Ropporin has also been identified as a binding protein of rhophilin, which is a putative downstream target for Rho, a small GTPase [4]. These findings suggest that AKAP3 compartmentalizes some signaling pathways including Rho and A-kinase into the ribs. Based on the preferential localization on the rib, GAPDS may contribute to the signaling associated with AKAP3.
Concerning the other FS proteins, AKAP4, spermatogenic cell-specific type 1 hexokinase (HK1-S) and calcium-binding tyrosine phosphorylation-regulated protein (CABYR) are distributed throughout the entire FS [8, 12, 13]. Post-embedding immunogold method was used for the localization of AKAP4 and CABYR, but pre-embedding immunoperoxidase staining was applied for the localization of HK1-S, hence the precise localization of the enzyme is unknown. Ropporin and rhophilin have been shown to be distributed on the inner and outer surfaces of FS by pre-embedding immunogold labeling [4]. However, in view of the permeability of the probe, the precise localization of these proteins within the structure remains undetermined. To our knowledge, the localization of the other FS components has not been studied at the electron microscopic level.
The present study indicates that rat GAPDS is expressed and is incorporated into FS during the final steps 16–19 of late spermiogenesis. Our data support a recent report which roughly describes the expression of GAPDS in rat spermatids but unfortunately does not describe the first appearance of GAPDS on the flagellum in developing spermatids [22]. By comparing the expression and incorporation of rat GAPDS into the flagellum with the biogenesis of FS and the expression of other FS components in the rat, the present results enable us to document the temporal expression of GAPDS in association with the assembly process of the FS components. The rib anlagen first emerges at step 11 and the end of the morphological development of the ribs is estimated to occur around step 15 during rat spermiogenesis in a conventional electron microscopic study [6]. Concomitantly with the morphological development, the incorporation of newly synthesized proteins into the ribs is exclusively observed during steps 11–15 [6]. AKAP4 is first detected in the cytoplasm of step-9 spermatids and in the flagellum of step-10 spermatids, and the maximum immunoreactivity in the flagellum is shown in step-15 and later spermatids in rat [15]. Taken together, a large portion of the FS components is assembled within the period until step 15. Based on the expression period, GAPDS is incorporated into the ribs after the major cytoskeletal architecture of the ribs has already been constructed. Similarly, SPTRX-2 is expressed during the final steps 15–19 of spermiogenesis in the rat [11]. Also, the mouse glycolytic enzymes, GAPDS and HK1-S, show a similar temporal expression during the final steps of spermiogenesis [3, 12]. Although the data are still limited, these findings suggest that the non-structural FS components with enzymatic activities, such as GAPDS, HK1-S and SPTRX-2, are more likely to be expressed during the later steps of spermiogenesis after the bulk of FS components has already been assembled to form the cytoskeletal framework.
Between patients with dysplasia of FS (DFS) and normal controls, no quantitative or qualitative differences in either AKAP4 or AKAP3 have yet been reported [19]. This suggests that there are factors causing DFS other than mutation in genes encoding the structural proteins, such as AKAPs. Alternatively, the mutation in genes affecting the expression, transport, and packaging of FS components is assumed to be responsible for the DFS. The present study addressed the precise stage of the assembly of a glycolytic enzyme, GAPDS, into FS during spermiogenesis, indicating that there are factors regulating the sequential event of the FS formation. The accumulation of further data on the assembly of each FS component will help to identify the precise factors regulating the sequential assembly, leading to a better understanding of the molecular and genetic basis of the normal flagellar assembly process as well as the underlying causes of DFS.
V. Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Special thanks are due to Yasunori Fujii for his excellent technical assistance. We gratefully acknowledge the helpful suggestions of Akio Inoue of Osaka University and Kazuo Kitamura of University of Miyazaki.
VI. References
- 1.Carr D. W., Fujita A., Stentz C. L., Liberty G. A., Olson G. E., Narumiya S. Identification of sperm-specific proteins that interact with A-kinase anchoring proteins in a manner similar to the type II regulatory subunit of PKA. J. Biol. Chem. 2001;276:17332–17338. doi: 10.1074/jbc.M011252200. [DOI] [PubMed] [Google Scholar]
- 2.Chemes H. E. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J. Androl. 2000;21:799–808. [PubMed] [Google Scholar]
- 3.Fenderson B. A., Toshimori K., Muller C. H., Lane T. F., Eddy E. M. Identification of a protein in the fibrous sheath of the sperm flagellum. Biol. Reprod. 1988;38:345–357. doi: 10.1095/biolreprod38.2.345. [DOI] [PubMed] [Google Scholar]
- 4.Fujita A., Nakamura K., Kato T., Watanabe N., Ishizaki T., Kimura K., Mizoguchi A., Narumiya S. Ropporin, a sperm-specific binding protein of rhophilin, that is localized in the fibrous sheath of sperm flagella. J. Cell Sci. 2000;113:103–112. doi: 10.1242/jcs.113.1.103. [DOI] [PubMed] [Google Scholar]
- 5.Hess R. A. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol. Reprod. 1990;43:525–542. doi: 10.1095/biolreprod43.3.525. [DOI] [PubMed] [Google Scholar]
- 6.Irons M. J., Clermont Y. Kinetics of fibrous sheath formation in the rat spermatid. Am. J. Anat. 1982;165:121–130. doi: 10.1002/aja.1001650204. [DOI] [PubMed] [Google Scholar]
- 7.Jackson H., Partington M., Fox B. W. Effect of “Busulphan” (“Myleran”) on the spermatogenic cell population of the rat testis. Nature. 1962;194:1184–1185. doi: 10.1038/1941184b0. [DOI] [PubMed] [Google Scholar]
- 8.Johnson L. R., Foster J. A., Haig-Ladewig L., VanScoy H., Rubin C. S., Moss S. B., Gerton G. L. Assembly of AKAP82, a protein kinase A anchor protein, into the fibrous sheath of mouse sperm. Dev. Biol. 1997;192:340–350. doi: 10.1006/dbio.1997.8767. [DOI] [PubMed] [Google Scholar]
- 9.Mandal A., Naaby-Hansen S., Wolkowicz M. J., Klotz K., Shetty J., Retief J. D., Coonrod S. A., Kinter M., Sherman N., Cesar F., Flickinger C. J., Herr J. C. FSP95, a testis-specific 95-kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol. Reprod. 1999;61:1184–1197. doi: 10.1095/biolreprod61.5.1184. [DOI] [PubMed] [Google Scholar]
- 10.Miki K., Qu W., Goulding E. H., Willis W. D., Bunch D. O., Strader L. F., Perreault S. D., Eddy E. M., O’Brien D. A. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. U S A. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda-Vizuete A., Tsang K., Yu Y., Jimenez A., Pelto-Huikko M., Flickinger C. J., Sutovsky P., Oko R. Cloning and developmental analysis of murid spermatid-specific thioredoxin-2 (SPTRX-2), a novel sperm fibrous sheath protein and autoantigen. J. Biol. Chem. 2003;278:44874–44885. doi: 10.1074/jbc.M305475200. [DOI] [PubMed] [Google Scholar]
- 12.Mori C., Nakamura N., Welch J. E., Gotoh H., Goulding E. H., Fujioka M., Eddy E. M. Mouse spermatogenic cell-specific type 1 hexokinase (mHk1-s) transcripts are expressed by alternative splicing from the mHk1 gene and the HK1-S protein is localized mainly in the sperm tail. Mol. Reprod. Dev. 1998;49:374–385. doi: 10.1002/(SICI)1098-2795(199804)49:4<374::AID-MRD4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Naaby-Hansen S., Mandal A., Wolkowicz M. J., Sen B., Westbrook V. A., Shetty J., Coonrod S. A., Klotz K. L., Kim Y. H., Bush L. A., Flickinger C. J., Herr J. C. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev. Biol. 2002;242:236–254. doi: 10.1006/dbio.2001.0527. [DOI] [PubMed] [Google Scholar]
- 14.Nomura E., Katsuta K., Ueda T., Toriyama M., Mori T., Inagaki N. Acid-labile surfactant improves in-sodium dodecyl sulfate polyacrylamide gel protein digestion for matrix-assisted laser desorption/ionization mass spectrometric peptide mapping. J. Mass Spectrom. 2004;39:202–207. doi: 10.1002/jms.578. [DOI] [PubMed] [Google Scholar]
- 15.Oko R., Clermont Y. Light microscopic immunocytochemical study of fibrous sheath and outer dense fiber formation in the rat spermatid. Anat. Rec. 1989;225:46–55. doi: 10.1002/ar.1092250108. [DOI] [PubMed] [Google Scholar]
- 16.Roth J., Taatjes D. J., Tokuyasu K. T. Contrasting of Lowicryl K4M thin sections. Histochemistry. 1990;95:123–136. doi: 10.1007/BF00266584. [DOI] [PubMed] [Google Scholar]
- 17.Tanii I., Yoshinaga K., Toshimori K. A 90-kDa glycoprotein exhibited stage-specific expression in meiotic germ cells in rat testes. Histochem. Cell Biol. 2000;114:181–189. doi: 10.1007/s004180000178. [DOI] [PubMed] [Google Scholar]
- 18.Tanii I., Oh-oka T., Yoshinaga K., Toshimori K. A mouse acrosomal cortical matrix protein, MC41, has ZP2-binding activity and forms a complex with a 75-kDa serine protease. Dev. Biol. 2001;238:332–341. doi: 10.1006/dbio.2001.0380. [DOI] [PubMed] [Google Scholar]
- 19.Turner R. M., Musse M. P., Mandal A., Klotz K., Jayes F. C., Herr J. C., Gerton G. L., Moss S. B., Chemes H. E. Molecular genetic analysis of two human sperm fibrous sheath proteins, AKAP4 and AKAP3, in men with dysplasia of the fibrous sheath. J. Androl. 2001;22:302–315. [PubMed] [Google Scholar]
- 20.Welch J. E., Schatte E. C., O’Brien D. A., Eddy E. M. Expression of a glyceraldehyde 3-phosphate dehydrogenase gene specific to mouse spermatogenic cells. Biol. Reprod. 1992;46:869–878. doi: 10.1095/biolreprod46.5.869. [DOI] [PubMed] [Google Scholar]
- 21.Welch J. E., Brown P. L., O’Brien D. A., Magyar P. L., Bunch D. O., Mori C., Eddy E. M. Human glyceraldehyde 3-phosphate dehydrogenase-2 gene is expressed specifically in spermatogenic cells. J. Androl. 2000;21:328–338. [PubMed] [Google Scholar]
- 22.Welch J. E., Barbee R. R., Magyar P. L., Bunch D. O., O’Brien D. A. Expression of the spermatogenic cell-specific glyceraldehyde 3-phosphate dehydrogenase (GAPDS) in rat testis. Mol. Reprod. Dev. 2006;73:1052–1060. doi: 10.1002/mrd.20235. [DOI] [PubMed] [Google Scholar]