Abstract
Terminal Schwann cells (TSCs) that cover motor neuron terminals, are known to play an important role in maintaining neuromuscular junctions, as well as in the repair process after nerve injury. However, the molecular characteristics of TSCs remain unknown, because of the difficulties in analyzing them due to their paucity. By using our previously reported method of selectively and efficiently collecting TSCs, we have analyzed the difference in expression patterns of lysophospholipid (LPL) receptor genes (LPA1, LPA2, LPA3, S1P1, S1P2, S1P3, S1P4, and S1P5) between TSCs and myelinating Schwann cells (MSCs). LPL, which includes lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), is the bioactive lipid that induces a myriad of cellular responses through specific members of G-protein coupled receptors for LPA. It turned out that LPA3 was expressed only in TSCs, whereas S1P1 was expressed in TSCs and skeletal muscle, but not in MSCs. Other types of LPL receptor genes, including LPA1, S1P2, S1P3, S1P4, were expressed in both types of Schwann cells. None of the LPL receptor gene family showed MSCs-specific expression.
Keywords: terminal Schwann cell, myelinating Schwann cell, lysophospholipid receptors, single cell RT-PCR, in situ hybridization
I. Introduction
Lysophospholipid (LPL) is a phospholipid signaling molecule and its functions that have been reported up to now include cell proliferation, myogenesis, stress fiber formation, neurite retraction and transient increase of C2+ [1, 2, 7]. The effects of LPL are mediated through distinct G-protein coupled receptors that are encoded by a different gene family. The first lysophosphatidic acid (LPA) receptor gene identified was the “ventricular zone gene-1 (vzg-1/LPA1)”, which was abundantly expressed in the ventricular zone of the embryonic cerebral cortex [3]. Recent studies demonstrated that LPA is related to the up-regulation of myelin-specific protein, morphological changes, and survival of Schwann cells (SCs) [22, 24]. Especially, Weiner and Chun demonstrated that LPA1 was expressed by postnatal SCs, and that LPA promotes SC survival via LPA1 activation and a pathway including Gi, PI3K (phosphoinositide-3-kinase) and Akt [23].
In the peripheral nervous system, SCs play important roles in the axonal regeneration following nerve injury. It is well known that there are two types of SCs, myelinating Schwann cells (MSCs) and terminal Schwann cells (TSCs). TSCs are located at neuromuscular junctions (NMJs) and the number of TSCs is usually 2–6 cells at individual NMJs [15]. Following partial denervation, processes of TSCs extend to the neighboring NMJ and induce the axonal regeneration from the neighboring NMJ [16–18]. On the other hand, MSCs extend, migrate, and proliferate in the same axonal tube after nerve injury [19, 20]. These differences in post-injury reaction between TSCs and MSCs may reflect the physiological significance of each type of SCs. However, the analysis of TSCs is difficult due to the paucity and the intricacy of collecting TSCs. We have previously devised a novel method of obtaining TSCs at the single cell level and found a TSC-specific gene, Herp, by applying PCR-differential display [13].
In order to elucidate the molecular characteristics of TSCs when compared to MSCs, we have studied the expressions of LPL receptors by single cell RT-PCR and in situ hybridization.
II. Materials and Methods
Animals
Adult Wistar rats (9- to 10-week-old female) were purchased from a breeder (SLC, Japan) 4 days prior to treatment, and maintained in a temperature- and humidity-controlled animal facility with a 12:12-hr light:dark cycle. The studies were approved by the Kyoto Prefectural University of Medicine Institutional Animal Care and Use Committee and all the experiments were carried out in full compliance with the Rules and Regulations for the Experimental Use and Care of Laboratory Animals at Kyoto Prefectural University of Medicine.
Preparation of total RNA from skeletal muscle and Schwann cells, and of genomic DNA
Total RNAs from soleus muscle and kidney were extracted and purified with RNeasy Mini kit (Qiagen, USA) according to the manufacturer’s instruction. Total RNAs from terminal Schwann cells and myelinating Schwann cells were prepared as previously described [13]. Briefly, single terminal Schwann cell was isolated as follows. The soleus muscle was dissected out from the animals after deep anesthesia by intraperitoneal injection of sodium pentobarbital. The muscle was trimmed in a central portion at the entry point of the sciatic nerve branch and embedded in a low-melting temperature agarose gel. Cross sections, 500 µm in thickness, of the muscle were prepared with a microslicer. The sections that include the nerve branch were selected for further studies. NMJs were stained with a fluorescent dye, 4-(4-diethylaminostyryl)-N-methyl-pyridinium iodide (4-Di-2-ASP, Molecular Probes, USA). After enzymatic treatment for cell dissociation, individual cells were collected carefully with microcapillaries under an epifluorescent stereomicroscope (Nikon, Japan) and transferred into small tubes containing the cell lysis buffer in Strataprep total RNA microprep kit. Myelinating Schwann cells were prepared from the sciatic nerves of adult Wistar rats. Sciatic nerves, free of perineurium and epineurium, were incubated with collagenase and trypsin to obtain dissociated cells. The cells were collected and transferred into the lysis buffer by using microcapillaries under conventional phase contrast microscope. After adding yeast tRNA (50 ng) to the above cell lysates, total RNA from each single cell was purified according to the instruction manual in the kit. Genomic DNA from kidney was purified with DNeasy tissue kit (Qiagen, USA) according to the manufacturer’s instruction.
cDNA synthesis
Total cDNAs from each type of single cell were synthesized and amplified using SMART cDNA synthesis kit (Clontech, USA) as previously described [4, 14]. The optimized number of thermal cycling was set at 18. Under these conditions, each amplified total cDNA fraction was obtained as moderately strong smears ranging from 0.2 to 4.0 kb with a scattering of several bright bands. Gene expression of Herp (a marker of terminal Schwann cell), S-100β, and GAPDH in each cDNA fraction was confirmed by PCR.
First strand cDNAs from soleus muscle and kidney were reverse-transcribed from 5 µg of DNase I-treated total RNA using random hexamers and Superscript II RNase H− (Invitrogen, USA).
PCR amplification of the genes of LPL receptor family from the cDNAs and genomic DNA
PCR using the amplified cDNAs, the first strand cDNA, and the genomic DNA as templates, was carried out with ExTaq DNA polymerase (TaKaRa Bio, Japan).
For the PCR reactions, sets of primers corresponding to each gene of LPA receptor family were used: 5'-CACTAACCAATGGCAGTATTTGTC-3' and 5'-CTGGCTTAGGCCAAACCACATAA-3' (LPA1), 5'-AGCCTGCTTGTCTTCCTRCTCAT-3' and 5'-TAAAGGGTGGAGTCCATCAG-3' (LPA2), 5'-TACATGCTACACACACGGGTGTA-3' and 5'-TAGCGATCTCCTCATGCTCGTA-3' (LPA3), 5'-GCCTAAGGACTATGCTGCTGTAA-3' and 5'-GAGTGTGACCAAGGACAGTCATA-3' (S1P1), 5'-CGGAGGCACTGACTAATCAGATT-3' and 5'-TCCCAGCACTCAGGACACAGTTA-3' (S1P2), 5'-AACCACAACTCCGAGAGATCCAT-3' and 5'-TTGAAGAGGATGGAGCACTCCTT-3' (S1P3), 5'-TRCTSAASACSGTGCTGATGAT-3' and 5'-CKGCTGCGGAAGGAGTAGATGA-3' (R=A/G, K=G/T: S1P4), 5'-CGTGTCCTGTGCTTCTGCAA-3' and 5'-CTGCAAACTGTTGGAGGAGTCTT-3' (S1P5).
Each PCR products were size-fractionated with 2% agarose gel electrophoresis followed by visualization with ethidium bromide. 2-log Ladder DNA size marker (New England BioLab, USA) was used as a size marker.
Sequencing
PCR products were cloned into pCRII TOPO plasmid DNA (Invitrogen, USA). Nucleotide sequence of each product was determined using Big Dye terminator cycle sequencing kit (v1.1) (Applied Biosystems, USA) and PRIZM310 Genetic Analyzer (Applied Biosystems, USA). Homology search was performed with BLAST 2.0 software program for the databases of GenBank, EMBL, DDBJ, and PDB.
In situ hybridization
Animals were deeply anesthetized with sodium pentobarbital and were transcardially perfused with 4% paraformaldehyde in phosphate buffered saline. Soleus muscles were dissected out and embedded in a low-melting temperature agarose gel. Longitudinal sections of the muscle, 200 µm thick, were prepared with a microslicer.
The PCR-amplified cDNA fragment of rat LPA3 (nt 1661–2111, GenBank NM_023969) was cloned into pGEM-T Easy plasmid DNA (Promega, USA). Digoxigenin-labeled cRNA probes were obtained by in vitro transcription using the plasmid as template. Sense- and antisense-strand probes were synthesized using SP6- and T7 RNA polymerase (Roche Diagnostics, Switzerland), respectively.
In situ hybridization was carried out according to the whole mount in situ hybridization protocol as described elsewhere [5, 13] using alkaline phosphatase-labeled anti-DIG antibody (Fab)2 fragment (Roche Diagnostics, Switzerland) and NBT/BCIP.
III. Results
In order to study whether each member of LPL receptor family is expressed in a cell-type-specific manner, we have analyzed the total cDNAs prepared from TSCs, MSCs and skeletal muscle, respectively, by PCR amplification with each gene-specific primer.
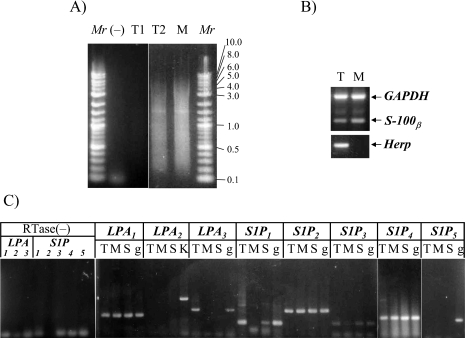
For this purpose, we collected each type of Schwann cells and purified their total RNAs followed by first strand cDNA synthesis and PCR amplification of total cDNAs. As shown in Figure 1A, we obtained total cDNAs fractions from each type of Schwann cell. Some of the amplified cDNAs fractions were insufficient (such as T1 in Fig. 1A). Such total cDNAs fractions were not used for the following analysis. Then, the expression of three genes, including Herp (a marker of terminal Schwann cell, as demonstrated in [13]), S-100β (a marker of Schwann cell) and GAPDH (a housekeeping gene) in each cDNA fractions was confirmed by PCR (as shown in Fig. 1B). The total cDNAs fractions expressing three genes mentioned above were used for analyzing the expression of LPL receptor mRNAs.
Fig. 1.
(A) Representative results of the size distribution of total cDNAs amplified from terminal Schwann cells and myelinating Schwann cells. T1 is of a failure in amplification for total cDNAs from terminal Schwann cells. T2 shows a successful amplification for total cDNAs from terminal Schwann cells. (−) denotes reverse transcriptase-omitted reaction as a negative control. (B) cDNA amplification of GAPDH, S100β and Herp from each single cell-derived total cDNA fraction. (C) Comparison of each LPL receptor mRNA expression among TSCs, MSCs and skeletal muscles, using each gene-specific primer set. RTase(−) indicates negative controls: results of amplification using each gene-specific primer set and reverse transcriptase-omitted total cDNAs fraction. Mr, DNA size marker; T, terminal Schwann cells; M, myelinating Schwann cells; S, skeletal muscles; K, kidney; g, rat kidney genomic DNA.
We compared the pattern of mRNA expression of LPL receptor family genes between terminal Schwann cells and myelinating Schwann cells. PCR reactions were carried out using each set of the family member gene-specific primers and the total cDNAs prepared as described above. Amplification target sequence of each gene except for LPA2 was included within a single exon of each gene. Rat genomic DNA, therefore, was used as a positive control template for each PCR reaction. For LPA2, however, rat kidney cDNAs were used as a positive control. Because each primer for LPA2 is located in different exons of the gene, the amplification condition of PCR conducted for cDNA template was not applicable to genomic DNA template. Hence, cDNAs from adult rat kidney where LPA2 mRNA is known to be expressed was used as the positive control template. On the other hand, reverse transcriptase-omitted reaction product as shown in Fig. 1A) was used as a negative control template.
As shown in Figure 1C, LPA3 was expressed only in TSCs, whereas S1P1 was expressed in TSCs and skeletal muscle, but not in MSCs. Neither LPA2 nor S1P5 was detectable in either type of Schwann cell. Other types of LPL receptor genes, including LPA1, S1P2, S1P3, and S1P4, were expressed in both types of Schwann cells. None of the LPL receptor family genes showed MSC-specific expression.
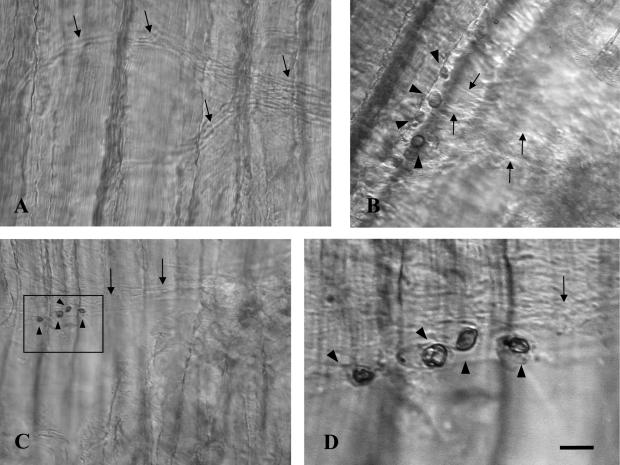
To confirm whether LPA3 was expressed selectively in TSCs, we have performed in situ hybridization in NMJs of adult rat soleus. In situ hybridization was carried out for the longitudinal sections of adult rat soleus muscle, according to a whole mount in situ hybridization protocol. With antisense probe, strong signals were detected specifically in the cell bodies located on top of motor nerve terminals, but not in nerve terminals themselves, axons, or postsynaptic areas (Fig. 2B, C). No signal was detected using sense probe as a negative control (Fig. 2A).
Fig. 2.
In situ hybridization in adult rat soleus using LPA3 sense cRNA probe as a negative control (A) and LPA3 antisense cRNA probe (B–D). Cell bodies of terminal Schwann cells on the rat soleus were stained (black arrowheads in B, C, D). Unstained axons were visualized under a differential interference contrast microscope (black arrows). D is a higher magnification image of the area indicated by a rectangle in C. Longitudinal and horizontal stripes were muscle fibers. Bar=50 µm (A), 20 µm (B), 40 µm (C), and 10 µm (D).
IV. Discussion
The present study demonstrated the specific expression of LPA3 in TSCs present in NMJs of adult rat soleus, with single-cell based RT-PCR as well as in situ hybridization. We have also demonstrated by RT-PCR that none of the LPL receptor family genes showed MSCs-specific expression.
In the peripheral nervous system, there are two types of Schwann cells, TSCs and MSCs. Both types of Schwann cells share the same lineage of cell origin, that is, neural crest cells, but behave in a different manner after maturation [8]. MSCs are known to contribute to axonal outgrowth and guidance in the regeneration process following nerve injury [6, 19]. On the other hand, TSCs appear to play pivotal roles in maintaining the structure and function of NMJs as well as in the repair process [6, 21]. Recently, Son and Thompson and Son et al. demonstrated that processes of TSCs extend to the neighboring NMJ following partial denervation and the axonal regeneration was observed along the processes of TSCs [16, 18]. The mode of nerve sprouting at NMJ is different from that at the nodes of Ranvier following injury. The regenerating sprouts which emerge from the nodes of Ranvier always extend distally within the basal lamina of MSCs [14]. On the other hand, the terminal nerve sprouts penetrate their own Schwann cell basal lamina tubes and grow toward the neighboring NMJ [17]. These different modes of nerve sprouting may reflect different properties of Schwann cell resources, i.e., TSCs and MSCs. However, there are few studies on the comparative aspects of physiological as well as pathological characteristics of TSCs and MSCs. We previously devised a method of isolating and collecting TSCs [13] and this method enabled us to analyze gene expression in TSCs by RT-PCR. Here, we have applied a similar method to elucidate the expression of LPL receptor family genes in TSCs and compared the expression between TSCs and MSCs.
LPL is a phospholipid signaling molecule that has intercellular signaling pathways and has the broad biological functions such as cell proliferation, myogenesis, stress fiber formation, neurite retraction and increase of Ca2+ [9–12]. The LPL receptor family of G-protein coupled receptors comprises two groups: LPA receptors and sphingosine-1-phosphate (S1P) receptors. LPA receptors consist of LPA1 (Edg-2/lpA1/vzg-1/rec1.3), LPA2 (Edg-4/lpA2) and LPA3 (Edg-7/lpA3), and S1P receptors include S1P1 (Edg-1/lpB1), S1P2 (Edg-5/lpB2/AGR16/H218), S1P3 (Edg-3/lpB3), S1P4 (Edg-6/lpB4), and S1P5 (Edg-8/lpB5/nrg-1). Weiner and Chun studied the expression of LPL receptors in Schwann cells (SC) in vivo and in vitro using total RNA by Northern blot analysis, and reported that LPA1 was expressed at high level by SCs in vivo and in vitro, whereas S1P1 was only expressed in SCs in vivo. Neither LPA2 nor S1P2 was detectable, although S1P3 was expressed in SCs in vivo and in vitro [23]. On the contrary, we were unable to detect the expression of S1P1 in isolated MSCs. This difference might be caused by the sampling method, because Weiner and Chun recognized expression of S1P1in vivo. Considering the fact that MSCs and TSCs behave differently after nerve injury, it would be worth studying changes of expression of each member of LPL gene family after nerve injury. Those studies are currently underway.
In conclusion, we studied the differential expression of LPL receptor gene family in TSCs and MSCs at single cell-based RT-PCR and found that LPA3 is specifically expressed in TSCs as confirmed by in situ hybridization. This differential expression of LPL receptors in TSCs and MSCs may underlie the behavior of each type of Schwann cells after nerve injury.
V. Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (to SO) and by a Grant-in-Aid for Young Scientists (B) (to TY) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
VI. References
- 1.Chun J., Weiner J. A., Fukushima N., Contos J. J., Zhang G., Kimura Y., Dubin A., Ishii I., Hecht J. H., Akita C., Kaushal D. Neurobiology of receptor-mediated lysophospholipid signaling. From the first lysophospholipid receptor to roles in nervous system function and development. Ann. N Y Acad. Sci. 2000;905:110–117. doi: 10.1111/j.1749-6632.2000.tb06543.x. [DOI] [PubMed] [Google Scholar]
- 2.Fukushima N., Kimura Y., Chun J. A single receptor encoded by vzg-1/LPA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc. Natl. Acad. Sci. U S A. 1998;95:6151–6156. doi: 10.1073/pnas.95.11.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht J. H., Weiner J. A., Post S. R., Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota R., Itoh K., Yaoi T., Bamba H., Uno T., Hisa Y., Fushiki S. Molecular changes in neurons of rat nucleus ambiguous after axotomy, as revealed by a novel method of in vivo fluorescence neuronal labeling combined with single-cell RT-PCR. Acta Histochem. Cytochem. 2005;38:229–235. [Google Scholar]
- 5.Hogan B., Beddington R., Constantini F., Lacy E. Manipulating the mouse embryo. In “In Situ Hybridization of Whole Mount Embryos with RNA Probes”, 2nd ed. Cold Spring Harbor Laboratory Press; New York: 1994. pp. 352–367. [Google Scholar]
- 6.Ide C., Tohyama K., Yokota R., Nitatori T., Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Gonzalez M. I., Meinkoth J. L., Field J., Kazanietz M. G., Tennekoon G. I. Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J. Biol. Chem. 2003;278:9585–9591. doi: 10.1074/jbc.M213244200. [DOI] [PubMed] [Google Scholar]
- 8.Love F. M., Thompson W. J. Schwann cells proliferate at rat neuromuscular junctions during development and regeneration. J. Neurosci. 1998;18:9376–9385. doi: 10.1523/JNEUROSCI.18-22-09376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moolenaar W. H. Lysophosphatidic acid, a multifunctional phospholipid messenger. J. Biol. Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 10.Moolenaar W. H. Lysophosphatidic acid signalling. Curr. Opin. Cell Biol. 1995;7:203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 11.Moolenaar W. H., Kranenburg O., Postma F. R., Zondag G. C. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr. Opin. Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- 12.Moolenaar W. H. Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 13.Oda R., Yaoi T., Okajima S., Kobashi H., Kubo T., Fushiki S. A novel marker for terminal Schwann cells, homocysteine-responsive ER-resident protein, as isolated by a single cell PCR-differential display. Biochem. Biophys. Res. Commun. 2003;308:872–877. doi: 10.1016/s0006-291x(03)01499-2. [DOI] [PubMed] [Google Scholar]
- 14.Okajima S., Mizoguchi A., Masutani M., Tomatsuri M., Tamai K., Hirasawa Y., Ide C. Synaptophysin immunocytochemistry in the regenerating sprouts from the nodes of Ranvier in injured rat sciatic nerve. Brain Res. 1993;631:133–136. doi: 10.1016/0006-8993(93)91198-2. [DOI] [PubMed] [Google Scholar]
- 15.O’Malley J. P., Waran M. T., Balice-Gordon R. J. In vivo observations of terminal Schwann cells at normal, denervated, and reinnervated mouse neuromuscular junctions. J. Neurobiol. 1999;38:270–286. [PubMed] [Google Scholar]
- 16.Son Y. J., Thompson W. J. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 17.Son Y. J., Thompson W. J. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995;14:133–141. doi: 10.1016/0896-6273(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 18.Son Y. J., Trachtenberg J. T., Thompson W. J. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci. 1996;19:280–285. doi: 10.1016/S0166-2236(96)10032-1. [DOI] [PubMed] [Google Scholar]
- 19.Torigoe K., Tanaka H. F., Takahashi A., Awaya A., Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp. Neurol. 1996;137:301–308. doi: 10.1006/exnr.1996.0030. [DOI] [PubMed] [Google Scholar]
- 20.Torigoe K., Lundborg G. Selective inhibition of early axonal regeneration by myelin-associated glycoprotein. Exp. Neurol. 1998;150:254–262. doi: 10.1006/exnr.1997.6775. [DOI] [PubMed] [Google Scholar]
- 21.Trachtenberg J. T., Thompson W. J. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379:174–177. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- 22.Weiner J. A., Hecht J. H., Chun J. Lysophosphatidic acid receptor gene vzg-1/LPA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J. Comp. Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- 23.Weiner J. A., Chun J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc. Natl. Acad. Sci. U S A. 1999;96:5233–5238. doi: 10.1073/pnas.96.9.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner J. A., Fukushima N., Contos J. J., Scherer S. S., Chun J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J. Neurosci. 2001;21:7069–7078. doi: 10.1523/JNEUROSCI.21-18-07069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]