Abstract
Hard tissue decalcification can cause variation in the constituent protein characteristics. This paper describes a method of preparating of frozen mouse head sections so as to clearly observe the nature of the constituent proteins. Frozen sections of various green fluorescent protein (GFP) transgenic mouse heads were prepared using the film method developed by Kawamoto and Shimizu. This method made specimen dissection without decalcification possible, wherein GFP was clearly observed in an undamaged state. Conversely, using the same method with decalcification made GFP observation in the transgenic mouse head difficult. This new method is suitable for observing GFP marked cells, enabling us to follow the transplanted GFP marked cells within frozen head sections.
Keywords: green fluorescent protein (GFP), film method, hard tissue, decalcification
I. Introduction
The head is one of the most difficult anatomical parts to section and analyze histologically, as it consists of tissues of varying solidity such as teeth, bones and muscles. Teeth and bones are by far the hardest tissues, hence decalcification is necessary. Decalcification requires chemical reagents for softening, but this is both time consuming and very harmful, destroying the constituents of the decalcified head and often preventing the target constituents, such as proteins, from being detected [6, 11, 13].
There have been several attempts to make frozen thin sections from these complex tissue samples without decalcification [5]. Two methods, such as coating the cut surface with a polymer and supporting the section with a sheet of wet paper, were adopted for hard samples. Unfortunately, these methods cannot be used for water soluble materials [1, 15]. Another method, which supports the section with a pressure-sensitive adhesive tape, proves too difficult [12].
To overcome these problems we applied the film method developed by Kawamoto and Shimizu [4], which does not require decalcification. The mouse head section is supported by film and glue, avoiding decomposition that usually occurs due to the differing tissue solidities. This film method is applicable to frozen sections since it enables us to clearly observe the nature of the proteins [5].
Green fluorescent protein (GFP) was first identified as a fluorescence protein from the jellyfish in 1962 [9]. Since the GFP cDNA was analyzed, it has been widely used on various biological phenomena, due to its easy observation. GFP, even in viable cells, can be visualized by light emission without complicated preparation [10, 14]. This desirable characteristic makes GFP a useful protein marker in various biological experiments.
For example, GFP is generally used as a marker for DNA transfection in viable cell cultures; thus, GFP fluorescence assists researchers in visualizing the desired DNA-transfected cells [2]. GFP is also used to visual the localization of target proteins fused and expressing GFP, since this visualization technique discloses the internal events of living cells [3].
Furthermore, transgenic animals, whose genome contains the cDNA of GFP, enables investigators to use the GFP-marked transplanted organs or cells and follow their development in the recipient [7]. The GFP transgenic mouse (GFP mouse) was the first mammal created with GFP cDNA in the genome [8]. Consequently, the GFP mouse has been used as a donor in transplantation and regeneration experiments [7].
In order to visualize GFP in the transgenic mouse, the mouse head was embedded in glycol methacrylate after paraformaldehyde fixation. During decalcification, GFP-marked cells would lose their GFP fluorescence due to its instability in water and hydrophilic solvents [7].
In this study we prepared and observed GFP mouse head sections using the film method to visualize GFP fluorescence, without complicated procedures such as decalcification or plastic embedding.
II. Materials and Methods
Sections of GFP mouse heads using the film method
Twelve- to fifteen-week-old GFP mice (C57BL/6-TgN) were euthanized by cervical dislocation and their heads were dissected. The specimens were then frozen in hexane cooled with solid carbon dioxide. The frozen samples were immersed in 5% carboxymethyl cellulose (CMC) gel for ten minutes and completely frozen with the CMC gel in cooled hexane. Next, the frozen blocks were fastened to the cryomicrotome (CM 3050S IV; Leica Instruments, Germany) in the cryochamber (−25°C) and trimmed with a disposable tungsten carbide blade (Jung TC-65A, 35° angle; Leica Instruments) having a clearance angle of 15°. The trimmed surface was covered with a Cryofilm (FINETEC, Japan) using a brush to remove air bubbles behind the film. The sample was then cut at 5 to 7 µm thickness and dried at room temperature. Afterwards, the film was soaked briefly in ethanol and xylene (within 10 sec for each step) and mounted on a glass slide so that the sampling side faced upward.
Histological staining
The sections were washed under running water for 60 sec and stained with Mayer’s hematoxylin solution for 30 sec. Next, the stained sections were washed and stained with Eosin Y (Muto Pure Chemicals, Japan) solution for 30 sec. Finally, after another wash, the sections were mounted on glass slides with 30% glycerin.
Sample decalcification
The fresh sections were fixed with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) at room temperature for 12 hr and then decalcified with K-CX (Fujisawa Pharmaceutical, Japan) solution for 12 hr. Preparation of frozen samples and sections were identical to the methods described above.
GFP fluorescence examination
To observe the GFP fluorescence of mouse head sections, the sections were prepared as described above excluding the staining step (Figs. 1A, 2B and 2D). The film containing sample was removed from the glass slide and mounted again on another new slide to make the sample side close to the slide with 30% glycerin. Finally, the film was covered with a glass coverslip using Entellan new (MERCK, Germany) to prevent the film from wrinkling. GFP fluorescence was observed under a fluorescence microscope (Olympus, Japan). The GFP excitation beam wavelengths were between 470 and 490 nm and an emission filter, which permits the passage of 510 nm wavelengths, were used for observation.
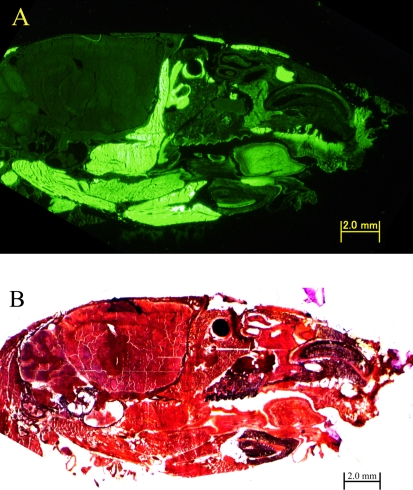
Fig. 1.
GFP mouse head sections prepared using the film method. 20-µm-thick sections of 14-week-old GFP mouse, ×20. (A) Fluorescence in a GFP mouse head section prepared by the film method. (B) Light microscopy of a hematoxylin-eosin stained GFP mouse head section prepared using the film method.
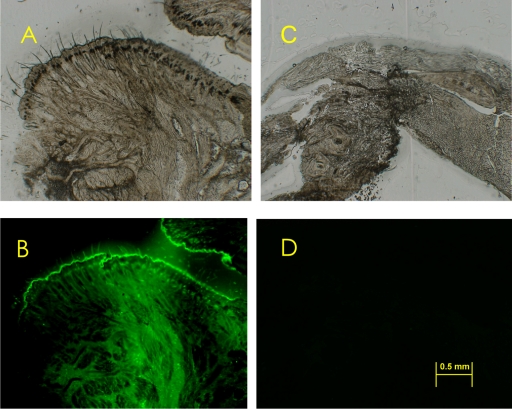
Fig. 2.
Effect of decalcification on GFP fluorescence. 20-µm-thick sections of a 14-week-old GFP mouse, ×80. (A) Light microscopy of a non-stained GFP mouse nose tip section prepared with the film method without decalcification. (B) Fluorescence of the same section used in (A). (C) Light microscopy of a non-stained chin of GFP mouse head section prepared by the film method with decalcification. (D) Fluorescence of the same section used in (C).
III. Results and Discussion
Sections of GFP mouse heads using the film method
Sections of the GFP mouse head made using the film method, clearly showed GFP fluorescence (Fig. 1). Because the GFP transgenic mouse used in this study was created to express GFP in all of the tissues [8], with the exception of erythrocytes and hair, we concluded that the green fluorescence in Figure 1A showed GFP expression. The conventional methods without decalcification described in the introduction would not be applicable to these sections of the GFP mouse head because of the GFP-characteristic instability in water and hydrophilic solvents. Conversely, this film method enables the frozen sections to be prepared easily and rapidly. Cellular GFP from the mouse head could be fixed with ethanol, such that the fluorescence of GFP was clearly displayed. Therefore, this film method is suitable for observing GFP fluorescence in the frozen sections without decalcification.
Comparison between decalcified and frozen sections
The film method makes satisfactory frozen sections of hard samples such as the mouse head without decalcification, since decalcification is time consuming and often damaging to tissue proteins. To illustrate these disadvantages, a decalcified GFP mouse head sample was prepared and protein damage was compared to a sample prepared using the film method (Fig. 2). As a result, GFP mouse head decalcified by K-CX solution, which was composed of chelating reagent and hydrochloric acid, lost its GFP fluorescence. On the other hand, GFP fluorescence in the head sections prepared by the film method was clearly observed. The film method is also effective for making flat sections of the mouse head, which is a mixture of hard organs like teeth and bones with soft organs such as muscles and tendons. Clearly the film method is very useful for making both microscopic and macroscopic sections of head samples and for observing the GFP proteins located within.
Application of film method
The film method is suitable for observing GFP fluorescence of hard samples like the head; thus, this method has the potential for various applications. For example, the film method is useful for chasing donor cells that are labeled with GFP in an acceptor body. This method, therefore, can be applied to regenerative medical research.
In order to apply this method to tissue development and regenerative medical research, however, the film method procedure needs adjusting. For instance, since this film method can prepare 2- to 50-µm-thick fresh-frozen sections, section thickness should be modified to reflect the experimental purpose. Specifically, Kawamoto and Shimizu recommend 5- to 10-µm-thick sections for macroscopic examination and 2- to 5-µm-thick sections for microscopic examination [4]. This method proves to be an effective tool for the study of tissue development, particularly in tissue regeneration research of bones and teeth.
IV. Acknowledgments
The authors would like to thank the staff of Division of Drug and Structural Research, Life Science Research Center, University of Toyama and FINETEC Co., Ltd. for their technical advice on the film method. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), Japan.
V. References
- 1.Aaron J. E., Carter D. H. Rapid preparation of fresh-frozen undecalcified bone for histological and histochemical analysis. J. Histochem. Cytochem. 1987;35:361–369. doi: 10.1177/35.3.2434557. [DOI] [PubMed] [Google Scholar]
- 2.Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin P. C. GFP biofluorescence: imaging gene expression and protein dynamics in living cells. Design considerations for a fluorescence imaging laboratory. Methods Cell Biol. 1999;58:343–367. [PubMed] [Google Scholar]
- 4.Kawamoto T., Shimizu M. A method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissues. Histochem. Cell Biol. 2000;113:331–339. doi: 10.1007/s004180000149. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003;66:123–143. doi: 10.1679/aohc.66.123. [DOI] [PubMed] [Google Scholar]
- 6.Mullink H., Henzen-Logmans S. C., Tadema T. M., Mol J. J., Meijer C. J. Influence of fixation and decalcification on the immunohistochemical staining of cell-specific markers in paraffin-embedded human bone biopsies. J. Histochem. Cytochem. 1985;33:1103–1109. doi: 10.1177/33.11.2414361. [DOI] [PubMed] [Google Scholar]
- 7.Ohta H., Yomogida K., Dohmae K., Nishimune Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 8.Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 9.Shimomura O., Johnson F. H., Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- 10.Shimomura O. Discovery of green fluorescent protein. In “Green Fluorescent Protein: Properties, Applications and Protocols”, ed. by M. Chalfie and S. R. Kain. Wiley-Liss; New York: 1998. pp. 3–15. [Google Scholar]
- 11.Takeshita N., Kuwahara S., Shirasuga H., Akiba M., Nagai N. The influence of decalcifying solution on immunoperoxidase staining (PAP) in paraffin sections. Jpn. J. Oral Biol. 1983;25:1134–1135. [Google Scholar]
- 12.Ullberg S., Larsson B. Whole-body autoradiography. Methods Enzymol. 1981;77:64–80. doi: 10.1016/s0076-6879(81)77012-5. [DOI] [PubMed] [Google Scholar]
- 13.Wakisaka S. [Immunohistochemical study on substance P in rat molar pulp and periodontal tissues. 1. Effect of decalcifying agents on substance P-like immunoreactivity] Osaka Daigaku Shigaku Zasshi. 1986;31:203–208. [PubMed] [Google Scholar]
- 14.Ward W. W. Biochemical and physical properties of green fluorescent protein. In “Green Fluorescent Protein, Applications and Protocols”, ed. by M. Chalfie and S. R. Kain. Wiley-Liss; New York: 1998. pp. 45–75. [Google Scholar]
- 15.Watanabe M., Kihara T., Shimada M., Kurimoto K. Preparation and stain of whole-body sections. Cell. Mol. Biol. Include. Cyto. Enzymol. 1978;23:311–315. [PubMed] [Google Scholar]