Abstract
Dysfunction of tight junctions (TJs), located at the most apical part of the intestinal epithelium, is believed to result in various complications in intestinal disease. However, the behaviors of multiple kinds of TJ proteins during ischemia-reperfusion injury are not understood in detail. To determine changes in expression and localization of TJ proteins during intestinal-barrier recovery, we induced intestinal ischemia-reperfusion injury in rats, measured mucosa-to-blood permeability of fluorescein isothiocyanate-dextran-4 kDa, and compared it with spatiotemporal changes of ZO-1, occludin, and claudin-1, -2, -3, -4, and -5 by immunoconfocal microscopy. At 1 hour post-reperfusion, villi were denuded and intestinal-barrier function was lost. From 6 to 24 hours post-reperfusion, villous epithelium was restored by cell migration, and barrier function together with reticular pattern expression of ZO-1, occludin, and claudin-1, -3, and -5, recovered time-dependently. To the contrary, after ischemia-reperfusion injury, the localized expression of claudin-2 and claudin-4 observed in the non-treated control was lost and replaced with broader expression from crypts to villi with increased basolateral claudin-4 expression in epithelial cells. These data demonstrated that recovery of intestinal barrier function is associated with expression of ZO-1, occludin, and claudin-1, -3, and -5, whereas claudin-2 and claudin-4 show unique changes in expression and localization.
Keywords: tight junctions, claudin-2, claudin-4, ischemia-reperfusion injury, barrier function
I. Introduction
The gastrointestinal mucosa is covered with one layer of epithelium. The intestinal epithelium serves as a protective barrier separating the luminal contents from the underlying tissue. Severe gut ischemia induces desquamation of the intestinal epithelium, increases the intestinal permeability, and in patients often causes fatal conditions that can result in sepsis and multiple organ failure. It has been proposed that increased intestinal permeability represents a loss of gut barrier function allowing bacteria from the lumen to cross the mucosal layer, thus causing local as well as systemic complications.
Tight junctions are the most apical component of intercellular junctional complexes, thereby establishing cell polarity and functioning as major determinants of epithelial barrier function [1]. Dynamic regulation of tight-junction function is fundamental to many physiological processes, and disruption of tight junctions drastically alters paracellular permeability and is a hallmark of many pathological states. Recent research efforts have disclosed molecular components of tight junctions; claudins and occludin mainly comprise the tight junction [3, 5, 6]. Claudins are integral transmembrane proteins of tight junctions, and at least 24 members of this family have been identified in mice/humans [15]. Among proteins constituting tight junctions, claudins are thought to play a crucial role in homeostasis in multicellular organisms. Claudins confer the barrier function as constituents of tight junction strands and directly participate in the transport of materials across simple epithelia through the paracellular pathway by adjusting the tightness and the selectivity of tight junction strands, but the biological role of each claudin is still incompletely understood. Various cells, organs, and tissue compartments have unique distributions of the various claudins, and according to recent reports, claudin-1, -2, -3, -4, and -5 are strongly expressed in the rat intestine [9, 13].
Reperfusion after ischemia leads to continued destruction of the intestinal epithelial structures, in part due to the generation of toxic oxygen free radicals and cytokines. Previous studies demonstrated an influence of cytokines and growth factors on tight junction function through selective and differential regulation in vitro [2, 9, 17]. Although it is quite likely that the functions of tight-junction proteins are dramatically changed during the recovery of intestinal epithelium from intestinal ischemia-reperfusion injury, only a few reports are available on changes of tight-junction protein expression, such as the one on occludin and ZO-1 expression in porcine intestine [11]. Moreover, the behavior of multiple claudins during recovery of barrier function in ischemia-reperfusion injury in vivo is still not understood in detail.
The purpose of this study was to determine changes in expression and localization of multiple kinds of tight-junction proteins during recovery of intestinal barrier function in ischemia-reperfusion injury. For this purpose, we induced intestinal ischemia-reperfusion in rats by occlusion of a branch of the superior mesenteric artery. Using this model, we measured the intestinal mucosa-to-blood permeability by intraluminal administration of fluorescein isothiocyanate (FITC)-dextran and analyzed the expression of ZO-1, occludin and claudin-1, -2, -3, -4, and -5 by immunoconfocal microscopy. Because we found that changes in claudin-4 expression were significantly different from those in other tight-junction proteins, we also performed morphometric analysis of claudin-4 expression.
II. Materials and Methods
Operative procedures
A total of 22 male Wistar rats age 8 to 9 weeks and weight 230–250 g were used. The animals were fasted for 12 hr before the procedure, with water allowed ad libitum. Each rat was anesthetized with an intraperitoneal injection of pentobarbital (8 mg/kg). After injection of anesthetic, the abdomen was exposed through a midline celiotomy and the terminal ileum was identified. The branch of the superior mesenteric artery supplying the terminal ileum was occluded with a microvascular clamp. To prevent collateral flow, 6 cm of terminal ileum was clamped proximally and distally with a large metallic clamp. Gauze pads were placed over the bowel and frequently moistened with warm phosphate-buffered saline (PBS). After 60 min of ischemia, reperfusion was started by removing both vascular and intestinal clamps and the clamped segment of ileum was marked with 4-0 silk sutures. The abdominal contents were replaced and abdomen was closed in a standard fashion. Rats were fasted after the procedure and received only water.
The measurement of intestinal mucosa-to-blood permeability was performed by the method previously described [10, 16]. Five animals per time point were re-anesthetized and midline laparotomy was performed at 1, 6, and 24 hr post-reperfusion. The segment of the ileum marked with 4-0 silk sutures was exposed and ligated at its distal and proximal ends with a vascular clamp and cannulated with an 18-G polyethylene tube. The lumen was gently washed with 10 ml of warmed PBS three times each, and 1 ml of 10 mM FITC-dextran-4 kDa (Sigma, St. Louis, MO) was injected into the intestinal lumen. Gauze pads were placed over the bowel, frequently moistened with warm PBS, and covered with aluminum foil to prevent photobleaching. Sixty min after injection of FITC-dextran-4 kDa into the intestinal lumen, 4-ml blood samples were collected from the inferior vena cava, and the segment of small intestine was ligated at both ends, filled with FITC-dextran-4 kDa, carefully removed and divided into five specimens. Three specimens from the central, proximal, and distal parts of the excised intestine, were immediately embedded in Tissue Tek OCT compound (Miles, Elkhart, IN) and frozen at −80°C. The other two specimens were fixed in 10% formalin and embedded in paraffin for morphologic study. Five rats were used for FITC-dextran-4 kDa permeability study without ischemia-reperfusion injury as a control group. Two animals were sacrificed at 48 hr post-reperfusion and the segment of small intestine was carefully removed and immediately embedded in Tissue Tek OCT compound and frozen at −80°C. All animals were sacrificed under deep anesthesia. This experimental procedure was approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine.
Measurement of intestinal mucosa-to-blood permeability
For determination of plasma FITC-dextran-4 kDa concentration, blood samples were centrifuged at 1,940 g for 15 min. The plasma was aspirated and diluted fivefold in PBS. The concentration of FITC-dextran-4 kDa was determined by fluorescence spectrophotometry, using a GENios XFLUO4 Version 4.40 (TECAN Inc, Research Triangle Park, NC). The excitation wavelength, excitation slit width, emission wavelength, emission slit width, and integration time were 465 nm, 10 nm, 535 nm, 10 nm, and 40 microseconds, respectively. The standard curve relating FITC-dextran-4 kDa concentration to fluorescence intensity was generated by adding known amounts of FITC-dextran-4 kDa to PBS. Concentrations of FITC-dextran-4 kDa in plasma were calculated by interpolation from the standard curve.
Immunohistochemistry
The applied primary antibody species, dilutions, and sources were rabbit anti-claudin-1 (1:200 dilution), rabbit anti-claudin-2 (1:400), rabbit anti-claudin-3 (1:400), mouse anti-claudin-4 (1:500), rabbit anti-claudin-5 (1:400), rabbit anti-occludin (1:200), and rabbit anti-ZO-1 (1:500), all from Zymed Laboratories, South San Francisco, CA. For immunohistochemical analysis, 8-µm-thick frozen sections of small intestine were cut using a cryostat perpendicular to the longitudinal axis of the tissue, mounted on glass slides, and air dried. The sections were fixed in absolute ethanol at 4°C for 30 min, and for 1 min in acetone, and washed three times in PBS, and nonspecific binding was blocked with PBS containing 5% skim milk for 30 min at room temperature. The sections were incubated for 60 min at 37°C with primary antibodies. After three washes in PBS, incubation with Alexa 488 goat anti-rabbit IgG (1:1000, Molecular Probes, Eugene, OR), or with Alexa 488 donkey anti-mouse IgG (1:1000, Molecular Probes) was performed for 30 min at 37°C. After final rinses in PBS, the sections were mounted in Vectorshield (Vector Laboratories, Burlingame, CA). All antibody dilutions were made using PBS containing 1% bovine serum albumin (Sigma) and 1% sodium azide with 0.1% Triton X-100. As negative controls, specimens were incubated in the absence of the primary antibodies. In addition, to monitor the DNA replication in the epithelial cells, bromodeoxyuridine (BrdU; Sigma) (10 mg/kg body weight) in 2.5 ml of PBS was injected i.p. into each animal 1 hr before it was killed. Frozen sections were treated with 2 N HCl for 1 hr and incubated with a sheep anti-BrdU polyclonal antibody (1:500 dilution, Molecular Probes) and then with Alexa 488 donkey anti-sheep IgG (1:1000 dilution, Molecular Probes). All nuclei were counterstained with propidium iodide.
Confocal laser scanning microscopy
After immunohistochemistry, the sections were observed with a confocal laser scanning microscope (FLUOVIEW system; Olympus, Tokyo, Japan) equipped with an oil immersion objective (Plan Apo X60, NA=1.4; Olympus) and a dry objective (Uplan Fl X20, NA=0.3; Olympus). An argon-krypton laser produced excitation bands at 488 nm for Alexa 488, and monochromatic light was used for differential interference contrast (DIC) images. Fluorescent images were collected with emission filters for 510–550 nm for Alexa 488, and simultaneous images (800×600 pixels, 12 bits each) of Alexa 488 label and DIC were acquired and stored. To visualize extended focus images of tight junctions, analysis was done by acquiring a 2-D image at each of more than seven adjacent focus planes that were 0.7 µm apart within the specimen by driving a focus motor.
Morphometric analysis of claudin-4 expression
For morphometric analysis, all aspects of tissue processing, immunolabeling, and imaging were strictly standardized. To exclude the possibility that variations in immunolabeling at different dates affected the morphometric data, one section each for control condition and 1, 6, and 24 hr post-reperfusion was included in a single immunolabeling run and all of the sections were analyzed at the same time. Digital images were collected by adjusting the sensitivity of the photomultiplier according to the fluorescence intensity of the positive control section from nontreated samples in each immunolabeling run, and the fluorescence intensities in the positive control sections were adjusted within the 4096-level scale. Section planes were obtained using ×60 objective lens and a zoom setting of 1.5 (800×600 pixels corresponds to 45000 µm2) under identical conditions, i.e., laser power=35 mW, pinhole size=100 µm, ND filter=20%, scanning speed=23.1 s/frame. Photomultiplier voltage was adjusted from 640 V to 680 V according to the intensity of the positive control image of the series to standardize the condition. For morphometric analysis of claudin-4 expression, a single optical plane was selected and four images of villi and four images of crypts were obtained from each section. Altogether 10 sections were used for each timepoint. The images were converted into 256 grayscale to quantify the fluorescence intensity of claudin-4 immunofluorescent staining and analyzed using the Scion image program (Scion Corporation, Frederick, MD).
The data for morphometric analysis of claudin-4 expression in the villi were expressed as both the total grayscale value of claudin-4 fluorescence per cell and the number of claudin-4-positive pixels per cell. Nine to thirteen epithelial cells, which were aligned but not overlapping each other, were manually enclosed to measure the claudin-4 expression. In the control samples, cells with clear staining were selected from the tips of the villi because claudin-4 expression was found to be restricted to the tips.
The data for morphometric analysis of claudin-4 expression in the crypts were expressed as both the total grayscale value of claudin-4 fluorescence per area of crypt, and the number of claudin-4-positive pixels per area of crypt because it was not possible to collect the image of the crypts without cell overlapping and it was very difficult to count the number of cells per crypt. Four separated crypts which were sliced as cross-sections and situated just above the muscularis mucosae were manually enclosed to measure the claudin-4 expression.
These investigations were performed at 0 (the control), 1, 6, and 24 hr post-reperfusion. However, at 1 hr post-reperfusion, the data were collected only from the crypts because of extensive epithelial cell loss from the apical surface and failure to observe tight-junction proteins.
Statistical analysis
Statistical analysis was performed using the Stat View program (SAS Institute, Inc., Cary, NC). Values for FITC-dextran-4 kDa concentration in plasma, grayscale value, and positive pixels in morphometric analysis of claudin-4 expression were calculated as mean±SD, and significant differences were defined using Scheffé’s test. Significant differences were defined as p<0.05.
III. Results
Histology
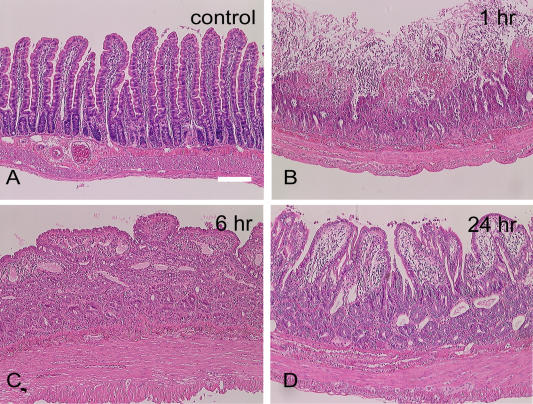
Representative hematoxylin-eosin-stained sections are depicted in Figure 1. At 1 hr post-reperfusion the ileum showed marked mucosal damage with villous necrosis with more than one-half luminal villous involvement (Fig. 1B). At 6 hr post-reperfusion, the mucosa was covered with flattened epithelial layers and villi were contracted, but small eroded areas were infrequently observed (Fig. 1C). At 24 hr post-reperfusion, the villi were increased in height but still contracted and blunted compared with control villi (Fig. 1D). There was no substantial interanimal variability in the degree of mucosal injury and recovery among all the 5 animals each at 1, 6 and 24 hr post-reperfusion.
Fig. 1.
Hematoxylin and eosin staining of (A) normal control rat ileum (not subjected to ischemia-reperfusion injury) and rat ileum at (B) 1 hr, (C) 6 hr, and (D) 24 hr post-reperfusion. Marked mucosal damage with necrosis of villi is seen at one hour post-reperfusion. Bar=100 µm.
In control samples, BrdU-labeled cells were restricted to the basal part of the crypt. At 6 hr post-reperfusion, BrdU-labeled cells were still restricted to the basal part of the crypt and none were observed in flattened surface epithelia. At 24 hr post-reperfusion, the distribution of BrdU-labeled cells was still restricted to the basal part of the crypt (data not shown).
Intestinal mucosa-to-blood permeability during ischemia-reperfusion injury
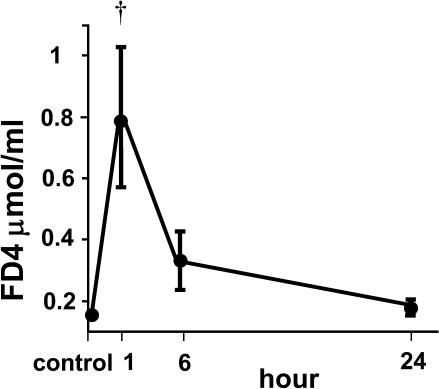
FITC-dextran-4 kDa concentrations in plasma samples obtained from rats at various times following ischemia-reperfusion injury are shown in Figure 2. The concentration was significantly increased at 1 hr post-reperfusion and thereafter showed a time-dependent decrease. At 24 hr post-reperfusion, there was no significant difference in FITC-dextran-4 kDa concentration compared with the control.
Fig. 2.
Intestinal mucosa-to-blood permeability measured by FITC-dextran-4 kDa (FD4) concentration in the plasma after ischemia-reperfusion injury. Control samples were taken from animals without ischemia-reperfusion injury. † p<0.01 compared with control and 6 and 24 hr post-reperfusion by Scheffé’s test.
Changes in tight-junction protein expression during ischemia-reperfusion injury
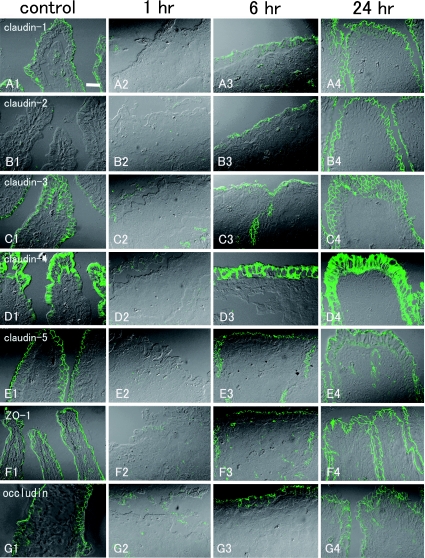
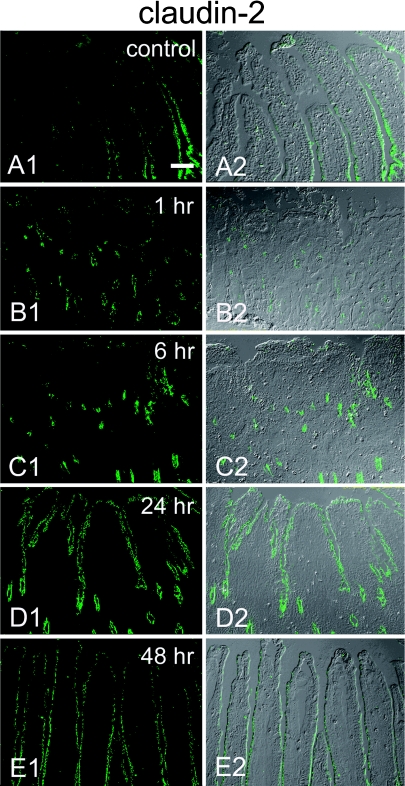
Immunohistochemistry using control sections of rat ileum demonstrated the expression of claudin-1, claudin-2, claudin-3, claudin-4, claudin-5, ZO-1, and occludin. Claudin-1, claudin-3, claudin-5, ZO-1, and occludin were expressed in linear fashion along the whole surface of the epithelial cells from the crypts to the villi with a typical reticular pattern (Fig. 3A1, C1, E1–G1). Claudin-2 showed a gradient of decreasing expression from crypts to villi, and no expression was observed on the tips of the villi even in extended focus images (Fig. 3B1, Fig. 4A1, A2). At 1 hr post-reperfusion, villi were denuded, lamina propria was exposed, and no tight-junction proteins were observed at the surface (Fig. 3A2–G2). At the crypts that survived ischemia-reperfusion injury, the reticular pattern distribution of claudin-1, claudin-2, claudin-3, claudin-5, ZO-1, and occludin remained at 1 hr post-reperfusion and showed no changes compared with the control samples (data not shown). At 6 hr post-reperfusion, apical surfaces were mostly covered with epithelial cells. Claudin-1, claudin-2, claudin-3, claudin-5, ZO-1, and occludin expression was observed in the recovering epithelium, but the reticular pattern was partly disrupted and irregular at the apical surfaces. Extended focus images of the tight-junction proteins showed spotty expression with only partly reticular pattern at the surface of the epithelium (Fig. 3A3–C3, E3–G3), whereas the reticular pattern of tight-junction protein expression was observed in the crypt. At 24 hr post-reperfusion, villi had recovered and the reticular pattern of distribution of ZO-1, occludin and claudin-1, -3, and -5 expression had also reverted to resemble that in the non-treated, control samples (Fig. 3A4, C4, E4–G4). In the crypt, the reticular pattern of tight-junction protein expression was observed. However, claudin-2 expression was observed on the villous surface but the decremental gradient from the crypts to the tips of the villi seen in the control samples was not observed (Fig. 3B4, Fig. 4D1, D2). The decremental gradient of claudin-2 similar to that noted in the control sample was observed at 48 hr post-reperfusion, but claudin-2 expression still remained at the tips of the villi (Fig. 4E1, E2). There was no substantial interanimal variability in the pattern of tight junction proteins expression among all the 5 animals each at 1, 6 and 24 hr post-reperfusion.
Fig. 3.
Extended focus images of immunohistochemical staining for tight-junction proteins in rat ileum during ischemia-reperfusion injury acquired with DIC imaging. Control samples: claudin-1 (A1), claudin-2 (B1), claudin-3 (C1), claudin-4 (D1), claudin-5 (E1), ZO-1 (F1), and occludin (G1). Claudin-1, -2, -3 and -5 and ZO-1 and occludin are expressed at the apical surface of the epithelium with reticular patterns. Claudin-4 is expressed at the apical surface of the epithelium; weak expression is also observed at the basolateral surfaces. At 1 hr post-reperfusion: [claudin-1 (A2), claudin-2 (B2), claudin-3 (C2), claudin-4 (D2), claudin-5 (E2), ZO-1 (F2), and occludin (G3)], no tight-junction protein expression is observed at the surface because villi are denuded. At 6 hr post-reperfusion: [claudin-1 (A3), claudin-2 (B3), claudin-3 (C3), claudin-4 (D3), claudin-5 (E3), ZO-1 (F3), and occludin (G3)], the reticular patterns of distribution of claudin-1, -2, -3 and -5 and ZO-1 and occludin are partly irregular and disrupted. Strong expression of claudin-4 is observed. At 24 hr post-reperfusion: [claudin-1 (A4), claudin-2 (B4), claudin-3 (C4), claudin-4 (D4), claudin-5 (E4), ZO-1 (F4) and occludin (G4)], villi have almost recovered and reticular patterns of distribution of claudin-1, -2, -3 and -5 and ZO-1 and occludin are observed at the apical surface of the epithelium. Claudin-4 is still extensively expressed. Bar=20 µm.
Fig. 4.
Low-magnification images showing immunohistochemical staining for claudin-2 obtained from a single confocal plane with/without DIC imaging. In the control sample (A1, A2), claudin-2 shows decremental gradient of expression from the basal part to the tips of the villi. No expression of claudin-2 is observed at the tips of the villi. At 1 hr post-reperfusion (B1, B2), no expression on the surface is observed because villi are denuded. At 6 hr post-reperfusion (C1, C2), expression is observed at the crypt and the basal part of the villi, weak at the surface epithelium. At 24 hr post-reperfusion (D1, D2), claudin-2 expression is observed from the tips of the villi to the base of the crypt. At 48 hr post-reperfusion (E1, E2), the decremental gradient of expression from crypts to villi is observed, but claudin-2 expression is still observed at the tips of the villi. Bar=60 µm.
Changes in claudin-4 expression during ischemia-reperfusion injury
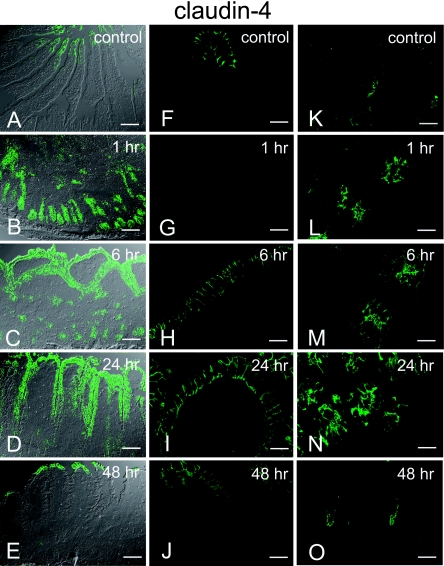
In the control sample, claudin-4 expression was restricted to the tips of the villi (Fig. 5A, F) and to the extreme basal part of the crypt (Fig. 5K). At the tips of villi, the expression of claudin-4 was distributed at lateral surfaces (Fig. 5F). Claudin-4 expression in the crypts was junctional and predominantly limited to the apical side of the cell-cell contact; basolateral expression was very weak (Fig. 5K). At 1 hr post-reperfusion, villi were lost due to mucosal damage and claudin-4 was not observed (Fig. 3D2, Fig. 5G). Claudin-4 expression remained confined to the crypt, and its fluorescence intensity appeared stronger than that in the control (Fig. 5B). Claudin-4 expression was observed not only at the apical but also at the lateral surfaces of the cell (Fig. 5L). At 6 hr post-reperfusion, strong claudin-4 expression was observed at the lateral surface of the cells in villi (Fig. 5H), and at the apical and lateral surfaces of the cells in the crypts (Fig. 5M). Compared with the control samples, the fluorescence intensity of claudin-4 expression appeared increased at both villi and crypts and the expression was not localized to the tips of the villi but was extensively distributed on the villous epithelia (Fig. 3D3, Fig. 5C, 5H, 5M). At 24 hr post-reperfusion, claudin-4 was still extensively expressed from the tips to the lower bases of the villi, with a decreasing gradient (Fig. 5D). Strong lateral expression was observed on the villi (Fig. 5I). Strong expression was also observed at the apical and lateral surfaces of the cell in the crypt (Fig. 5N). At 48 hr post-reperfusion, the expression of claudin-4 was again observed at the tips of the villi (Fig. 5E), and at the extreme basal part of the crypts (Fig. 5O). In the villi, claudin-4 was localized at the lateral surface of the cell, whereas in the crypts it was localized to the apical side of the cell-cell contact (Fig. 5O); this pattern was similar to that observed in the control.
Fig. 5.
Images of immunohistochemical staining for claudin-4 obtained from a single confocal plane acquired with/without DIC imaging. Lower magnification images: (A)–(E). High-magnification images of the villi: (F)–(J). High-magnification images of the crypt: (K)–(O). Control images: (A), (F), (K). Claudin-4 expression is restricted to the tips of the villi (A, F) and to the extreme basal part of the crypt preferentially limited to the apical side of the cell (K). At 1 hr post-reperfusion (B, G, L), no expression on the surface is observed because villi are denuded (G). Claudin-4 expression remains at the crypt base with apical and lateral expression (L). At 6 hr post-reperfusion (C, H, M), strong expression of claudin-4 is observed at the surface of the epithelium with lateral expression (H). The strong expression at the crypt persists (M). At 24 hr post-reperfusion (D, I, N), claudin-4 is still extensively expressed from the tips to the villi with a diminishing gradient (D). The lateral expression in the villi (I), and apical and lateral expression in the crypt (N) are still observed. At 48 hr post-reperfusion (E, J, O), expression of claudin-4 is restricted to the tips of the villi (E, J) and to the lower base of the crypt (O), which is similar to the expression pattern observed in the control sample. Bars=20 µm (A–E), 60 µm (F–O).
Morphometric analysis of claudin-4 expression
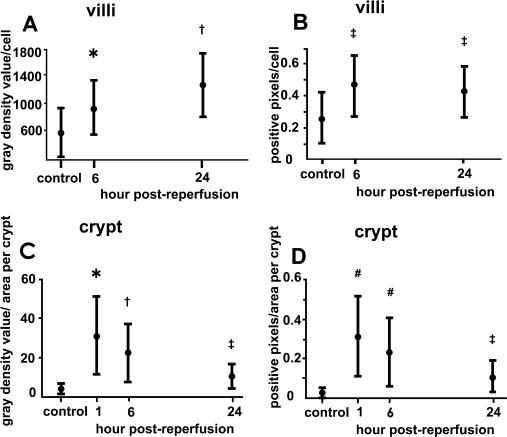
Because the changes in claudin-4 expression were significantly different from those in other tight-junction proteins, morphometric analysis of claudin-4 expression was performed. In the villi, the total gray density value per cell showed time-dependent increase until 24 hr post reperfusion (Fig. 6A). The increase of the total gray density value of claudin-4 fluorescence per cell indicates that claudin-4 expression level was increased time-dependently during the recovery of epithelium. The number of positive pixels per cell was increased at 6 hr post-reperfusion and kept at the increased level at 24 hr post-reperfusion, demonstrating that the area with claudin-4 expression in the cell was increased during the recovery of epithelium.
Fig. 6.
Morphometry of claudin-4 expression. (A) Total gray density value of claudin-4 immunolabeling per cell on villi. * p<0.05 compared with control and 24 hr post-reperfusion. † p<0.05 compared with control and 6 hr post-reperfusion. (B) The number of claudin-4-positive pixels per cell on villi in the binary image. ‡ p<0.05 compared with control. (C) Total gray density value of claudin-4 immunolabeling per area of crypt. (D) The number of claudin-4-positive pixels per area of crypt in the binary image. (C, D) * p<0.05 compared with control and 6 and 24 hr post-reperfusion. † p<0.05 compared with control, 1 hr, and 24 hr post-reperfusion. ‡ p<0.05 compared with 1 hr and 6 hr post-reperfusion. # p<0.05 compared with control and 24 hr post-reperfusion. Scheffé’s test.
In the crypts, both the total gray density value of claudin-4 fluorescence per area and the number of claudin-4-positive pixels in the binary image per area were increased at 1 hr post-reperfusion and thereafter gradually decreased (Fig. 6C, D). These results showed that claudin-4 expression level and area of expression in each crypt were the highest at 1 hr post-reperfusion and then decreased gradually, although they remained higher than in the control sample, during the recovery period.
IV. Discussion
The present study demonstrated that the expressions and localizations of multiple kinds of tight-junction proteins varied during recovery of intestinal barrier function in ischemia-reperfusion injury, i.e., the expression, especially with reticular pattern, of ZO-1, occludin and claudin-1, -3, and -5, was closely correlated with recovery of intestinal barrier function, whereas claudin-2 and claudin-4 showed unique changes in the expression.
In this study, the early (6 hr post-reperfusion) recovery of villous epithelium lost after ischemia-reperfusion injury is considered to have been effected mainly by migration of surviving epithelial cells and not by cell proliferation, for the reasons given below. Histological study of hematoxylin- and eosin-stained sections showed that, at 1 hr post-reperfusion, cells at crypts survived although villi were denuded and, at 6 hr, flattened epithelium covered villous mucosa. On the other hand, analysis for DNA replication demonstrated that at 6 hr post-reperfusion BrdU-labeling was present in the crypts but absent in flattened surface epithelia. These results are in accord with those shown by Park and Haglund [12], who concluded that rapid mucosal reconstitution occurred after intestinal ischemia primarily through cell migration based on findings that mitotic figures were observed only at the bottom of the crypt at 45 or 90 min of ischemia and at 6 hr of reperfusion.
The close correlations between the recovery of barrier function and expression of occludin and ZO-1 observed in our study are in good agreement with a recent report, showing a correlation between intestinal barrier function measured by trans epithelial resistance (TER) and expression of occludin and ZO-1 during mucosal recovery in ischemia-injured porcine ileum [11]. The present study revealed for the first time that in addition to occludin and ZO-1, the recovery of claudin-1, -3, and -5 expression was closely correlated with that of barrier function after ischemia-reperfusion injury in vivo.
We should remark that in our model, barrier function of the intestinal mucosa includes not only the integrity of tight junctions but also integrity of the villous structure. The loss of barrier function at 1 hr post-reperfusion is due largely to loss of the villous structure. At 6 hr post-reperfusion, most of the surface was covered with flattened epithelial layers although infrequently eroded small areas existed. From this observation, it seems reasonable to assume that the functional recovery of tight junctions is one of the important factors in the recovery of barrier function between 6 and 24 hr post-reperfusion. The reticular pattern of the tight junction protein ZO-1, occludin and claudin-1, -3, and -5 expression observed at 24 hr post-reperfusion may reflect the functional recovery of tight junctions.
In contrast to ZO-1, occludin and claudin-1, -3, and -5, claudin-4 was unique not only in the localization in the control ileum but also in changes of expression during ischemia-reperfusion injury. The present results of claudin-4 localization at lateral surface of the cells in the normal villi are consistent with those reported by Rahner et al. [13], which demonstrated the specificity of the claudin-4 antibody we used. In addition, we found that the restricted expression of claudin-4 at the tips of the villi and at the extreme basal part of the crypt observed in the control were lost and replaced by broad expression over the entire surface epithelium by 24 hr post-reperfusion. In particular, the preferential expression of claudin-4 at the apical surface of the cell-cell contact in the crypt was expanded to apico-lateral expression at from 1 hr to 6 hr post-reperfusion. On the other hand, in the villi, the lateral immunohistochemical expression increased at 6 hr post-reperfusion, and the expression remained strong until 24 hr post-reperfusion. Regarding the different patterns of changes of claudin-4 expression in the crypts and in the villi, we conjecture that the cells that survived the ischemia-reperfusion injury in the crypts were converted to overexpression of claudin-4 and migrated upwards to cover the apical surface. The villous epithelium would then be reconstructed when these cells with strong claudin-4 expression reach the apical surface, which would result in increased claudin-4 expression in the villous epithelium.
Among the several different effects of the increased and broadened expression of claudin-4 on the intestinal epithelium during ischemia-reperfusion injury that can be considered is the role of claudin-4 in the maintenance of barrier function. Although the role of claudin-4 in maintaining barrier function has been shown in several studies [14, 18], it is not likely that basolaterally expressed claudin-4 observed at the post-reperfusion period constitutes tight junction strands or that it is directly involved in the barrier function. Furuse et al. [8] reported that claudin-1 and claudin-4 were diffusely distributed, respectively, along the plasma membrane of keratinocytes from the stratum basale to the stratum granulosum, and in stratum granulosum, in mouse epidermis. They showed that these claudins did not constitute tight junction structure and that tracer injected into the dermis diffused freely through intercellular routes among these keratinocytes.
Our study suggests that claudin-4 overexpression plays an important role in cell migration which is essential at the early stages of epithelial recovery. Overexpression of claudin-4 was observed in rapidly migrating epithelial cells at the early stages of recovery, whereas at 48 hr post-reperfusion, when the structure of the villi and cell migration had returned to normal, overexpression was no longer observed.
We have shown that changes in claudin-2 expression are also unique to the period during recovery of epithelium. In the control sample, no claudin-2 expression was observed at the tips of the villi. However, claudin-2 expression was observed over the whole surface of the villi at 24 hr post-reperfusion. Our results for the claudin-2 expression in the control intestine are congruent with those reported by Escaffit et al. [4], who demonstrated that claudin-2 was detected in both crypts and villus cells of the small intestine but was restricted to undifferentiated crypt cells in the colon. They also showed that in the colonic Caco-2 cell model, claudin-2 was found to be present only in undifferentiated cells. Accordingly, the claudin-2 expression observed over the whole surface of the villi at 24 hr post-reperfusion in this study suggests that villous epithelial cells are not fully differentiated at 24 hr post-reperfusion.
Concerning the role of claudin-2 in barrier function, Furuse et al. [7] showed that the addition of claudin-2 markedly decreased the barrier function measured by TER in MDCK-1 cells that endogenously expressed claudin-1 and -4. Because they found significant reduction of TER but no difference in the paracellular flux of either FITC-dextran-4 kDa or -40 kDa after the addition of claudin-2, it is not likely that the claudin-2 expression observed over the entire surface of the villi at 24 hr post-reperfusion in this study had major effects on changes in the intestinal mucosa-to-blood permeability measured by FITC-dextran-40 kDa. In fact, at 24 hr post-reperfusion, there was no significant difference in plasma FITC-dextran-4 kDa concentration compared with the control. Nevertheless, we cannot deny the possibility that claudin-2 expression over the whole surface of the villi at 24 hr post-reperfusion affects tight junctional barrier function including TER.
In conclusion, we demonstrated that the expression, especially with reticular pattern, of multiple kinds of tight-junction proteins ZO-1, occludin and claudin-1, -3, and -5, was correlated with recovery of intestinal barrier function after ischemia-reperfusion injury. However, claudin-2 and claudin-4 undergo unique changes in expression during recovery of intestinal epithelium mediated by cell migration in ischemia-reperfusion injury. Evaluation of the role of tight-junction proteins, especially claudin-4 and claudin-2, may provide some insight into the barrier function and reparative process in the gastrointestinal tract. Furthermore, it would be of value to study what changes in tight-junction protein expression occur in humans, such as patients with ischemic and inflammatory bowel disease, with the goal of understanding the pathogenesis more clearly.
V. Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank Ramona Ratliff for advice on manuscript preparation.
VI. References
- 1.Anderson J. M., Van Itallie C. M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 2.Bruewer M., Luegering A., Kucharzik T., Parkos C. A., Madara J. L., Hopkins A. M., Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 3.Denker B. M., Nigam S. K. Molecular structure and assembly of the tight junction. Am. J. Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 4.Escaffit F., Boudreau F., Beaulieu J. F. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J. Cell. Physiol. 2005;203:15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- 5.Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuse M., Furuse K., Sasaki H., Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinugasa T., Sakaguchi T., Gu X., Reinecker H. C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 10.Kuebler J. F., Toth B., Rue L. W., Bland K. I., Chaudry I. H. Differential alterations in intestinal permeability after trauma-hemorrhage. J. Surg. Res. 2003;112:198–204. doi: 10.1016/s0022-4804(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 11.Little D., Dean R. A., Young K. M., McKane S. A., Martin L. D., Jones S. L., Blikslager A. T. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G46–G56. doi: 10.1152/ajpgi.00121.2002. [DOI] [PubMed] [Google Scholar]
- 12.Park P. O., Haglund U. Regeneration of small bowel mucosa after intestinal ischemia. Crit. Care Med. 1992;20:135–139. doi: 10.1097/00003246-199201000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Rahner C., Mitic L. L., Anderson J. M. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 14.Sonoda N., Furuse M., Sasaki H., Yonemura S., Katahira J., Horiguchi Y., Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukita S., Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr. Opin. Cell Biol. 2002;14:531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 16.Unno N., Wang H., Menconi M. J., Tytgat S. H., Larkin V., Smith M., Morin M. J., Chavez A., Hodin R. A., Fink M. P. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 17.Van Itallie C. M., Balda M. S., Anderson J. M. Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J. Cell Sci. 1995;108:1735–1742. doi: 10.1242/jcs.108.4.1735. [DOI] [PubMed] [Google Scholar]
- 18.Van Itallie C., Rahner C., Anderson J. M. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]