Abstract
NF-κB is regulated by inhibitor proteins (IκBs), which retain NF-κB in the cytoplasm. Signal-induced phosphorylation by the IκB-kinase complex containing the IκB-kinases 1 and 2 (IKK-1/2 or IKK-α/β) and subsequent degradation of the IκB proteins are prerequisites for NF-κB activation. Many signals induce NF-κB, one of them being oncogenic Raf kinase. We investigated whether NF-κB induction is critical for Raf-mediated transformation. Here, we demonstrate that inhibition of NF-κB interferes with transformation by the Raf-oncogene, and we characterized the mechanism of NF-κB induction by activated Raf kinase and the tumor promoter phorbol 12-myristate 13-acetate (PMA). NF-κB activation by PMA and Raf critically depends on the IκB-kinase complex, most notably on IKK-2. A major signaling pathway induced by Raf is the mitogenic cytoplasmic kinase cascade. However, different inhibitors of this cascade do not affect PMA- and Raf-mediated NF-κB activation. Raf does not phosphorylate the IκB-kinase proteins directly. Raf rather synergizes with another membrane shuttle kinase MEKK1, and Raf-mediated activation of NF-κB is blocked by a dominant negative form of MEKK1. These results suggest that Raf induction of NF-κB is relayed by MEKK1, but not by the classical mitogenic cytoplasmic kinase cascade.
The NF-κB/Rel transcription factor family consists of five mammalian family members (RelA, RelB, c-Rel, p105/NF-κB1, and p100/NF-κB2), which bind to DNA as homodimers or heterodimers (1, 2). The critical role of NF-κB family members for distinct cellular functions, such as cell proliferation, cytokine gene expression, cell differentiation, or protection from apoptosis, has been revealed by gene knockout experiments (3–7). Furthermore, there is good evidence for a role of NF-κB transcription factors in different pathophysiological processes, including cancer (2, 8). The binding of one of the three inhibitory proteins IκBα, IκBβ, or IκBɛ is responsible for sequestering the NF-κB/IκB complex in the cytoplasm (9). A wide variety of inducers trigger signal transduction cascades, which lead to the phosphorylation of specific serine residues in the NH2-terminal domains of the IκB proteins. Phosphorylated IκB proteins become polyubiquitinated and degraded by means of the proteasome pathway. NF-κB is released and migrates to the nucleus. Target genes regulated by NF-κB play important roles in immune, inflammatory, or stress responses, cell adhesion, and protection against apoptosis (2).
IκB degradation and, as a consequence, NF-κB activity can be induced in many cell types by different stimuli. Several parallel signal transduction pathways must exist, which all ultimately lead to IκB-kinase activation and IκB degradation. Among the best understood signaling pathways are the ones for the inflammatory cytokines tumor necrosis factor α (TNF-α) and IL-1 (10). In both cases, ligands bound to the receptors result in the recruitment of TRAF proteins (TRAF2 and TRAF6 for TNF-RI/TNF-RII and IL-1R, respectively) (11, 12). Two membrane shuttle kinases, MEKK1 and NIK (NF-κB-inducing kinase), are involved in relaying the signal to the IκB-kinase complex. TRAF proteins directly interact with NIK and thereby most likely activate NIK (10). In addition, RIP and IRAK protein kinases are critically involved in the signal transduction processes initiated by TNF-α and IL-1, respectively (13–16).
The multisubunit IκB-kinase complex contains two related cytokine inducible kinases, IKK-1 and IKK-2 (17–21). IKK-1 and IKK-2 directly phosphorylate IκB proteins with higher affinity for IκB proteins complexed with NF-κB (22). Two other components of the high molecular weight IκB-kinase complex have been identified: a protein called NEMO (NF-κB essential modulator)/IKKγ, which seems to be critical for association/function of this complex (23, 24), and IKAP, which serves as a scaffold protein for NIK, IKK-1, and IKK-2 (25). NIK and MEKK1 are able to phosphorylate members of the IκB-kinase complex in vivo and/or in vitro (26–28).
The Raf protein plays a critical role for regulating processes, such as cell proliferation, differentiation, and apoptosis. Raf functions as an effector kinase of Ras and is a crucial integrator in the mitogenic cytoplasmic protein kinase (MAPK) cascade (29). Most of its effects are mediated through direct phosphorylation of MEK, a dual specificity kinase, which itself phosphorylates and activates the extracellular signal-regulated kinase (ERK) proteins. The role of Raf in activating NF-κB has been less clear. Although Raf responsiveness of NF-κB-driven reporter constructs was shown by us (30) and others (31), the mechanisms proposed for the mode of action of Raf were controversial. In some cell types, Raf has been shown to activate NF-κB by induction of an autocrine loop (32, 33). However, there is evidence for a faster, more direct NF-κB activation by Raf indicative of an alternative signaling pathway.
Here, we have investigated the role of NF-κB activation for Raf-mediated cell transformation and the mechanism by which phorbol 12-myristate 13-acetate (PMA) and Raf induce NF-κB activity. We show that inhibition of NF-κB activation blocks transformation by Raf. The IκB-kinase complex, most prominently IKK-2, is critically involved in Raf- and PMA-mediated NF-κB induction. Raf activation of NF-κB is independent of the mitogenic kinase cascade, but depends on activation of MEKK1. This suggests that the IKK-complex represents a target of Raf kinase activation.
Materials and Methods
Plasmids and Antibodies.
Raf constructs BXB, BXB-CX, kinase dead BXB-CX(K375W), and the v-raf expression plasmid EHneo have been described (34). HA-BXB-DD was generated by site-directed mutagenesis. VSV-tagged wild type and mutant IKK-2 (35), MEK-mutant S217A (36), and kinase dead MEK-K97M (37) have been described. All inserts were cloned into the pcDNA3 vector (Invitrogen). pGex-IκBα (1–72) wild type and pGex-IκBα (1–72) mutant (AA), the luciferase reporter plasmids (3xκB.luc, 4 × 17.TATA, HSV-tk-luc) (38, 39), and the Gal-Elk expression vector have been described (40). Anti-VSV antibodies (clone P5D4) were purchased from Sigma; antisera against IKK-1, IKK-2, and ERK-2 were obtained from Santa Cruz Biotechnology.
Cell Culture and Transfection Experiments: Focus Assay.
Conditions for cell culture and electroporation of cells were as described (41). For transient transfections, 3 μg of the κB-dependent luciferase reporter (3xκB.luc) or 4 μg of 4 × 17.TATA reporter together with 1 μg of RSV-Lac-Z were used. The total amount of DNA was 25–30 μg DNA, and this was filled up with expression vectors. Typically, 10 μg of the appropriate expression vector and/or empty expression vectors were used. Samples were split in aliquots, and, 16 h after transfection, cells were stimulated appropriately. Cells were harvested 6 h later. Luciferase enzyme activity was determined, and β-galactosidase levels were used to normalize for differences in transfection efficiencies. Focus assays were performed as described (42). Average focus yield was 650 foci per μg EHneo DNA.
Immune-Complex Kinase Assays, Western Immunoblots, and EMSA.
Twenty-four hours after transfection, cells were washed in ice-cold PBS. For IKK activity assays, cells were lysed in Nonidet P-40 lysis buffer (43), and IKK proteins were immunoprecipitated with anti-VSV antibodies (tagged IKK2) or anti-IKK-2 antisera (endogenous IKK-2). To assay ERK activity, cells were lysed in RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) (34) and ERK-2 was precipitated with antihemagglutinin (HA) antibody (tagged ERK-2) or anti-ERK-2 antiserum (endogenous ERK-2). For IKK assays, 600 ng wild-type or mutant glutathione S-transferase (GST)-IκBα was used as substrate (23). Activity of ERK-2 was determined by using myelin basic protein as a substrate. Kinase activities were quantified by PhosphorImager analysis (44). Conditions for extract preparation, EMSA using a consensus NF-κB site, and Western immunoblots have been described (45, 46).
Preparation of GST-IKK-2 Fusion Protein and Active c-Raf from Sf9 Cells.
The GST-IKK-2 kinase domain fusion was obtained by cloning the N-terminal fragment (amino acids 1–291) of IKK-2 into pGEX-2T (Amersham Pharmacia). The fusion protein was expressed in Escherichia coli-Bl21 and purified (glutathion Sepharose beads followed by Mono Q chromatography). Purity of the eluted protein was at least 90% (SDS/PAGE, Coomassie staining). Sf9 insect cells were infected with a baculovirus expressing GST-RafΔ1–302YY340–341DD (GST-BXB-DD; P. Cohen, Dundee) at a multiplicity of infection of 5. Forty-eight hours after infection, cells were lysed and GST-BXB-DD was precipitated by using gluthation Sepharose beads. Purity of GST-BXB-YY340–341DD was ≈70% (SDS/PAGE, Coomassie staining).
Results
Transformation of Fibroblasts by v-raf Is Blocked by Inhibition of NF-κB Induction.
The observation that the Raf kinase can induce NF-κB prompted us to analyze whether NF-κB induction is critical for cell transformation by oncogenic Raf. NIH/3T3 fibroblasts were transfected with the v-Raf expression plasmid EHneo together with a 4-fold excess of the wild-type and kinase dead versions of IKK-2 (dn-IKK-2). DN-IKK-2 had previously been shown to function as a dominant negative regulator for NF-κB induction by TNF-α and other stimuli (19–21, 27). Foci of morphologically transformed cells were counted 14 days after transfection. As shown in Table 1, v-raf-induced transformation was unaffected by the expression of the wild-type form of IKK-2. However, expression of dn-IKK-2 dramatically reduced the focus yield. This suggests the requirement of NF-κB induction for v-raf-mediated transformation. Further details of the interference of raf-transformation by various inhibitors of NF-κB induction will be presented elsewhere. Here, we focus our interest on the elucidation of the mechanism by which Raf can induce NF-κB.
Table 1.
Effect of wild-type (WT) and kinase dead (KD) versions of the IKK-2 protein on v-Raf-induced transformation of NIH/3T3 cells
| Focus yield, % | |
|---|---|
| v-Raf + vector | 100 |
| v-Raf + IKK-2 (WT) | 111 ± 28 |
| v-Raf + IKK-2 (KD) | 13 ± 11 |
A total of 250 ng of EHneo (v-Raf expression plasmid) were cotransfected with the indicated IKK-2 expression plasmids (1 μg), and transformed foci were counted after 2 wk.
The IκB-Kinase Is Involved in Raf-Mediated NF-κB Induction.
The above observation indicated that IKK-2 is involved in Raf-mediated NF-κB induction. To determine the contribution of the IKK complex to PMA- and Raf-induced NF-κB activity, the effect of dn-IKK-2 was analyzed in Jurkat T cells. NF-κB activity was measured in a transcriptional readout system by using a κB-dependent reporter gene construct (38). This reporter was activated 15-fold by treatment of the cells with PMA and 25-fold by TNF-α. Cotransfection of dn-IKK-2 blocked the activation by both TNF-α and PMA (Fig. 1A).
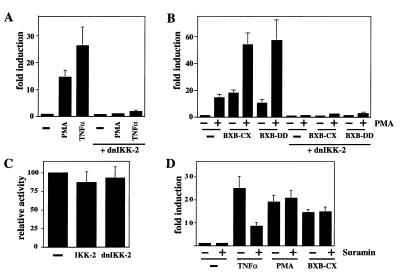
Figure 1.
Dominant-negative IKK-2 blocks PMA- and Raf-mediated NF-κB activation. (A) Jurkat T cells were cotransfected with a κB-dependent reporter (3xκB.luc) and either an expression vector for dn-IKK-2 or the empty expression vector (pcDNA3). Sixteen hours after transfection, cells were stimulated with PMA (20 ng/μl) or TNF-α (80 ng/μl) for 6 h as indicated. The luciferase activity for the noninduced cells cotransfected with pcDNA3 was arbitrarily set to 1, all other activities are given relative to this standard. (B) Jurkat T cells were cotransfected with 3xκB.luc and expression vectors for dn-IKK-2, Raf-BXB-CX, and Raf-BXB-DD as indicated. Stimulation with PMA was performed as above. (C) Jurkat cotransfection with the HSV-tk.luc reporter and expression vectors for either wild-type IKK-2 or dn-IKK-2. Expression level of the HSV-tk.luc construct (10 μg) cotransfected with the empty expression vector (pcDNA3) was set to 100%. (D) Jurkat cells were transfected with the κB-dependent reporter and active Raf as indicated. Sixteen hours post transfection, the cells were treated with suramin (1.5 mM f.c.). One hour later, PMA or TNF-α was added where indicated, and cells were harvested 6 h later.
We next tested the effect of dn-IKK-2 on two constitutively active versions of the Raf protein kinase, Raf-BXB-CX (34), and a Raf protein bearing two amino acids mimicking tyrosine phosphorylation (YY340–341DD). Phosphorylation of Raf at these tyrosines is involved in activation of Raf in T cells (29). Transfection of these Raf constructs into Jurkat cells induced NF-κB-dependent transcription to a similar level as PMA treatment (Fig. 1B). This activity could be further enhanced by inducing Raf-transfected cells with PMA. On cotransfection of dn-IKK-2, all activities were completely ablated. These results suggest that IKK-2, and therefore most likely the IKK complex, is involved in PMA- and Raf-induced NF-κB activity. The effects of the IKK-2/dn-IKK-2 cotransfections were specific for the NF-κB-dependent reporter because a constitutive promoter (the herpes virus thymidine kinase promoter) was not affected (Fig. 1C).
To distinguish between direct or indirect effects (i.e., by means of an autocrine loop) of PMA and Raf on NF-κB activation, we pretreated cells with suramin. Suramin, a heterocyclic polyanionic compound, interferes with ligand–receptor interactions on the cell surface (47–49). As expected, TNF-α-mediated NF-κB activity was reduced by treating the cells with suramin (Fig. 1D). In contrast, neither PMA-induced nor Raf-induced NF-κB activities were affected by this treatment (Fig. 1D). This argues against the induction of an autocrine loop by PMA and Raf and rather suggests a direct stimulation of NF-κB.
PMA and Raf Stimulate IκB-Kinase Activity.
Overexpression of the wild-type IKK-2 protein by itself was able to stimulate NF-κB-dependent transcription (Fig. 2A). When the constitutively active Raf construct was cotransfected with wild-type IKK-2, reporter gene activity was increased further. Similarly, PMA treatment resulted in an additional induction of IKK-2-mediated NF-κB activity. To address the question of whether Raf increases the IKK-2 kinase activity, immune-complex kinase assays were performed. Consistent with the observed reporter gene activity, overexpression of the wild-type IKK-2 protein alone resulted in a phosphorylation of the wild-type GST-IκBα substrate (Fig. 2B). To confirm the specificity of this phosphorylation, in vitro kinase reactions were also performed with a mutant GST-IκBα fusion protein, where both Ser-32 and Ser-36 were mutated to alanines. No phosphorylation was found with the mutant substrate.
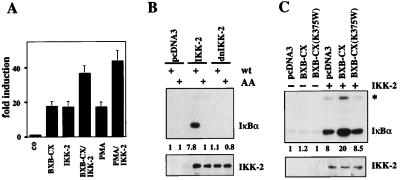
Figure 2.
Raf stimulates IKK-2 kinase activity. (A) IKK-2-induced NF-κB activity is enhanced by PMA and Raf. (A) Cotransfection of Jurkat T cells with 3xκB.luc and active Raf (Raf-BXB-CX) and/or wild-type IKK-2. Cells were stimulated with PMA as indicated. (B) IKK-2 overexpression results in constitutive phosphorylation of IκBα. (Upper) The autoradiograph of an immune-complex kinase assay using GST-IκBα as a substrate. Cells were transfected as above. VSV-IKK-2 was immunoprecipitated and incubated with either wild type (wt) or mutant IκBα (AA). Numbers indicate relative IκBα-specific kinase activities. Background activity in pcDNA3-transfected cell extracts was set to 1. (Lower) Control immunoblot analysis of immunoprecipitated IKK-2. (C) IKK-2 activity is increased by cotransfection of active Raf. Jurkat T cells were transfected with the indicated expression vectors. (Upper) An immune-complex kinase assay of immunoprecipitated VSV-IKK-2 with GST-IκBα as substrate. The asterisk marks the autophosphorylated IKK-2 protein. Numbers underneath the panel indicate the relative kinase activities. (Lower) Control immunoblot of precipitated IKK-2.
IKK-2 kinase activity was enhanced in extracts from cells cotransfected with activated Raf (Fig. 2C). The kinase activity of Raf is critical for this activation of IKK-2. When a mutant Raf bearing a mutation in the ATP binding site (BXB-CX-K375W) was cotransfected with IKK-2, the inactive Raf failed to stimulate IκBα-specific kinase activity of IKK-2 (Fig. 2C). Mutant Raf also failed to stimulate κB-dependent reporter gene activity (data not shown).
An additional band comigrating with the IKK-2 protein was in vitro phosphorylated with the immune complexes from IKK-2-transfected and IKK-2/Raf-BXB-CX-cotransfected cells (asterisk in Fig. 2C). This most likely represents autophosphorylation of IKK-2, as no such signal was observed in the cotransfections with the kinase-inactive IKK-2. Autophosphorylation of IKK-2 has been reported previously (20).
The Mitogenic Cytoplasmic Kinase Cascade Is Not Essential for NF-κB Induction by PMA and Raf.
The mitogenic signaling pathway involving Raf has been extensively studied in the past. Raf becomes activated by multiple extracellular signals, i.e., by means of Ras by ligands binding to tyrosine kinase receptors. The major downstream effector of Raf is the dual specificity kinase MEK, which phosphorylates and activates the ERK kinases. We therefore asked whether NF-κB activation by constitutively active Raf does involve this MEK–ERK cascade. The low molecular weight inhibitor PD98059 blocks MEK activation in many systems (50). PD98059 was added to Jurkat cells, and PMA-mediated NF-κB activity was analyzed. Untreated Jurkat cells show basal levels of nuclear NF-κB complexes that were strongly increased after TNF-α or PMA induction. Whereas TNF-α-induced NF-κB complexes were already present after 10 min, PMA induction of NF-κB was delayed and strong after a 30-min treatment (Fig. 3A). Addition of PD98059 did not decrease TNF-α or PMA-induced NF-κB DNA binding (Fig. 3A). Likewise, the PD98059 inhibitor did not reduce PMA- or Raf-stimulated κB-dependent reporter activity (data not shown).
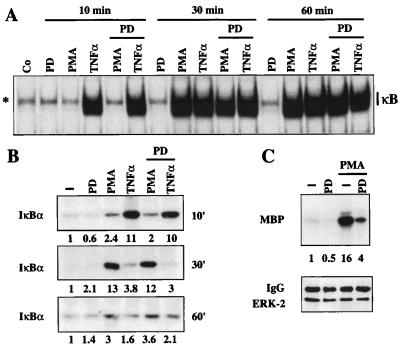
Figure 3.
The MEK inhibitor PD98059 does not interfere with PMA- or Raf-mediated NF-κB activation. (A) EMSA with whole-cell extracts (5 μg) from Jurkat cells treated as indicated and a κB-specific probe. The PD98059 (PD) inhibitor (50 μM) was added 2 h before PMA or TNF-α stimulation. The position of the induced NF-κB complexes as well as two nonspecific complexes (asterisks) are indicated. (B) PMA- and TNF-α-stimulated activity of the IKK complex are unaffected by the MEK inhibitor PD98059 (PD). Endogenous IKK activity was determined after stimulation of Jurkat cells in a time-dependent fashion using IκBα as a substrate (10-, 30-, and 60-min stimulations). Numbers indicate the IκBα-specific kinase activities. Activity in extracts from untreated cells was set to 1. (C) PMA stimulation of endogenous ERK-2 is inhibited by the MEK inhibitor PD98059. Jurkat T cells were stimulated with the indicated reagents for 30 min. Activity of endogenous ERK was determined in an immune-complex kinase assay using myelin basic protein (MBP) as a substrate. (Upper) MBP phosphorylation. (Lower) A Western blot for immunoprecipitated ERK-2. MBP-specific kinase activities are indicated by the numbers.
The lack of an effect of the PD98059 inhibitor on NF-κB induction was further investigated by analyzing the activity of the endogenous IKK-2 kinase. Again, PMA stimulated IKK-2 kinase activity with a delayed kinetics as compared with TNF-α (Fig. 3B). Maximal stimulation of IKK-2 by PMA was observed after 30 min. Neither TNF-α- nor PMA-induced IKK-2 kinase activity was significantly affected by the PD98059 inhibitor. In contrast, PD98059 strongly reduced the activity of the PMA-induced endogenous ERK-2 kinase (Fig. 3C). This shows that inhibition of the MEK activation selectively blocks the induction of ERK, a critical component of the mitogenic cytoplasmic kinase cascade, without affecting the NF-κB pathway.
These results suggested that activation of MEK is not critical for PMA- and Raf-mediated NF-κB induction. To further strengthen this suggestion, we analyzed the effect of dominant-negative MEK (dn-MEK) proteins. Two different MEK versions were used, a mutant that cannot be phosphorylated by Raf (MEK-S217A) and an ATP-binding mutant lacking kinase activity (MEK-K97 M). The effects of these dn-MEK proteins on the classical MAPK-cascade have been well documented in the past (36, 37). The dn-MEK expression vectors were transfected alone or together with active Raf into Jurkat cells, and κB-dependent reporter activity was scored. Neither of the two MEK mutants diminished Raf-induced transactivation of the NF-κB-dependent reporter (Fig. 4A). Identical results were obtained in HeLa cells, suggesting that the MAPK cascade-independent activation of NF-κB by Raf and PMA is not a Jurkat cell-specific phenomenon (data not shown). To corroborate the conclusion of a MEK-independent activation of the IκB-kinase complex by Raf, we investigated the effects of dominant-negative MEK on IKK-2 kinase activity. IKK-2 expression alone results in a measurable in vitro kinase activity, which was increased by coexpression of Raf. Cotransfection of the mutant MEK neither affected the constitutive activity nor did it affect Raf-induced IKK-2 activity (Fig. 4B). These results demonstrate that one downstream target of Raf, the MEK kinase, is not involved in Raf-mediated NF-κB activation.
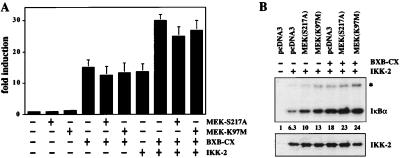
Figure 4.
Dominant-negative versions of MEK do not block Raf-mediated NF-κB activation. (A) Transient transfection of Jurkat cells with the 3xκB.luc reporter and expression vectors for active Raf (BXB-CX), wild-type IKK-2, and the two dominant-negative versions of MEK (MEK-S217A and MEK-K97 M, 15 μg each). (B) IKK-2 stimulation by active Raf (BXB-CX) is not inhibited by dominant-negative MEK (MEK-S217A). Jurkat T cells were transfected with the indicated plasmids as before. (Upper) An autoradiograph of an immune complex assay of IKK-2 activity using IκBα as a substrate. The band marked with an asterisk represents autophosphorylated IKK-2. Numbers indicate relative IKK-2 kinase activity. (Lower) Control immunoblot of immunoprecipitated IKK-2.
The MEK mutants did function as dominant negatives in two experimental systems. First, the dn-MEK proteins blocked PMA- and Raf-induced transcriptional activation by a GAL-ELK fusion protein, a known target of the Raf-MEK-ERK pathway (40, 51, 52). Second, dn-MEK proteins blocked Raf-induced ERK activity. Active Raf and HA-tagged ERK-1 were cotransfected into Jurkat cells, and kinase activity of immunoprecipitated ERK was assayed in vitro. Coexpressed dn-MEK efficiently inhibited the Raf-stimulated ERK activity (data not shown).
Stimulation of IκB-Kinase by Raf Involves MEKK1, Another Membrane Shuttle Kinase.
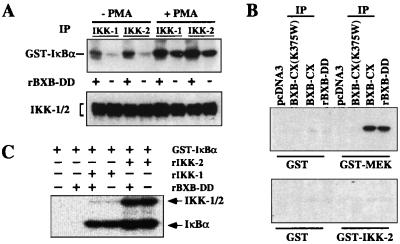
An important question was whether the observed stimulation of IKK kinase activity by Raf was direct or indirect. First, we asked whether recombinant Raf could stimulate IKK activity in vitro. Active (+) or heat inactivated (−) Raf kinase (RAF-BXB-DD) was added to crude cytosolic extracts from unstimulated or PMA-induced Jurkat cells. The endogenous IKK complex was subsequently immunoprecipitated and assayed for its ability to phosphorylate IκBα in vitro. Preincubation with active Raf-kinase resulted in a stimulation of IKK activities in all cases (Fig. 5A). As IKK-2 was coprecipitated with the IKK-1-specific antibody and IKK-1 coprecipitated with IKK-2 (Fig. 5A, Lower), the scored activity most likely was derived from the heterodimeric IKK-1/IKK-2 complex.
Figure 5.
Raf induces IKK activity in vitro, but does not directly phosphorylate the IKK proteins. (A) Cytosolic extracts (1 mg) from untreated or PMA-induced (30 min) Jurkat T cells were incubated with active (+) or inactivated (−) recombinant Raf-BXB-DD protein for 20 min at room temperature (f.c. 10 μM ATP). The endogenous IKK complex was immunoprecipitated with antibodies recognizing either IKK-1 or IKK-2 and tested in an in vitro kinase assay with GST-IκBα (Upper). (Lower) Control immunoblot of the immunoprecipitates with antibodies recognizing IKK-1 and IKK-2. (B) In vitro kinase assay with recombinant GST, GST-MEK, and GST-IKK-2 (1–291) as substrates. Jurkat T cells were transfected with HA-tagged Raf-BXB-CX, an HA-tagged kinase inactive mutant thereof, or the empty expression vector. Raf proteins were immunoprecipitated and tested in an immune-complex kinase assay with the indicated substrates. In addition, substrate proteins were also reacted with 5 μg recombinant Raf-BXB-DD purified from baculovirus-infected Sf9 cells. (C) Coupled in vitro kinase assay with GST-IκBα, 500 ng recombinant IKK-1, 50 ng recombinant IKK-2, and 5 μg recombinant Raf-BXB-DD as indicated. Positions of the phosphorylated IκBα and the autophosphorylated IKK proteins are marked.
These results prompted us to ask whether Raf directly phosphorylates the IKK proteins. We tested a recombinant GST-IKK-2 fusion protein containing the activation loop in an in vitro kinase assay with active Raf kinase. No phosphorylation of GST-IKK-2 was detectable, whereas the known Raf substrate MEK was readily phosphorylated under these conditions (Fig. 5B). Identical results were obtained with purified recombinant Raf protein (rBXB-DD) and immunoprecipitated Raf (BXB-CX) from transfected Jurkat cells. Similarly, when we used full-length recombinant IKK-1 and IKK-2 proteins (53) in a coupled kinase assay with the IκBα substrate, no stimulation or additional phosphorylation of IKK proteins was observed in the presence of Raf in vitro (Fig. 5C). Both IKK proteins showed some level of autophosphorylation, which was not affected by the addition of active Raf.
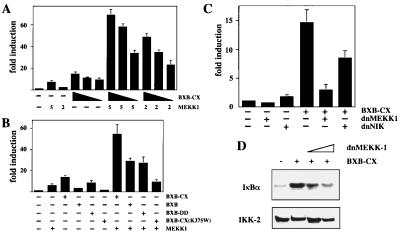
These observations suggested that the stimulation involves some intermediate(s). NIK and MEKK1 have been demonstrated to activate the IKK complex. To test whether these kinases would be involved, we asked whether active Raf could further induce MEKK1- or NIK-mediated NF-κB activity. Different amounts of MEKK1 expression vector were cotransfected with the κB-dependent reporter in the presence or absence of Raf-BXB-CX. Raf was able to synergistically enhance MEKK1-mediated activity at all concentrations tested (Fig. 6A). In contrast, no effect of Raf was detected in cotransfections with various amounts of NIK expression vector (data not shown). The synergism between Raf and MEKK1 was seen with the Raf-BXB-CX, the Raf-BXB-DD protein, and with Raf-BXB (Fig. 6B), which by itself has almost no NF-κB-inducing activity in T cells (34). A kinase-inactive Raf mutant (Raf-BXB-CX 375W) did not synergize with MEKK1, nor did it interfere with MEKK1-mediated NF-κB activation, suggesting that MEKK1 is downstream of the Raf action in this pathway (Fig. 6B). We then asked whether a dominant-negative version of MEKK1 would interfere with Raf-mediated NF-κB induction. Indeed, cotransfection of the dominant-negative MEKK1 construct efficiently inhibited Raf-induced κB-dependent reporter activity (Fig. 6C). A dominant-negative NIK version also reduced activity, albeit to a lower extent (Fig. 6C). To show that MEKK1 is critical for Raf-induced activation of the IKK complex, we cotransfected IKK-2 together with active Raf and increasing amounts of a dn-MEKK1 expression vector. Indeed, dn-MEKK1 could efficiently block the induction of IKK-2 kinase activity by Raf (Fig. 6D).
Figure 6.
MEKK1 is involved in Raf-mediated NF-κB activation. (A) Cotransfection of the 3xκB.luc reporter with either 5 or 2 μg of MEKK1 expression vector and decreasing amounts (10, 7, 5 μg) of Raf-BXB-CX, as indicated. Activity of the reporter cotransfected with the empty expression vectors is set to 1. (B) Cotransfection of the 3xκB.luc reporter with 5 μg MEKK1 and/or 10 μg of Raf-BXB-CX, Raf-BXB, Raf-BXB-DD, or Raf-BXB-CX (K375W), as indicated. (C) Cotransfection of the 3xκB.luc reporter with 10 μg Raf-BXB-CX and 10 μg of the dominant-negative versions of MEKK1 or NIK, as indicated. (D) Expression of dn-MEKK1 blocks Raf-induced IKK-2 activity. Jurkat T cells were cotransfected with 3 μg of VSV-tagged IKK-2 expression vector, 14 μg of RAF-BXB-CX, and 8 or 16 μg of dn-MEKK1, as indicated. IKK-2 was immunoprecipitated, and an in vitro kinase assay with GST-IκBα was performed. (Upper) The result of the in vitro kinase assay. (Lower) A Western immunoblot of the precipitated IKK-2.
Discussion
We have demonstrated that NF-κB induction is required for Raf-mediated transformation of fibroblast cells. Furthermore, we showed that Raf induces NF-κB by a pathway involving the MEKK1 protein and the IKK complex.
Earlier work had demonstrated that the activation of the mitogenic cytoplasmic kinase cascade by Raf is essential for Raf-mediated transformation (36, 42, 50). Our observation that Raf-induced transformation also critically depends on its ability to activate NF-κB suggests that Raf simultaneously stimulates minimally two independent signaling pathways to transform fibroblasts. This result is in agreement with recent reports showing that Ras-mediated transformation can also be efficiently blocked by inhibiting the NF-κB pathway (54, 55).
Earlier work had suggested a critical role of pp90rsk in the induction of NF-κB (56, 57). The pp90rsk kinase is activated by the mitogenic cytoplasmic kinase cascade. Our conclusion that the main signaling pathway for PMA- and Raf-mediated NF-κB activation is independent of pp90rsk is based on several observations. First, this activation is completely blocked by the dominant-negative IKK-2 protein. Second, inhibitors of the mitogenic kinase cascade, such as the PD98059 inhibitor and different dominant-negative forms of MEK, have no effect on Raf- and PMA-mediated NF-κB induction. This finding suggests that for PMA and Raf, as for many other stimuli, the IKK complex represents the main signal integrator. These results do not allow us to distinguish whether there is a single type of IKK complex integrating the signals from diverse stimuli or whether there are several biochemically distinct IKK complexes. They do suggest, however, that the IKK-2 protein is a common component of most complexes in vivo. Indeed, it has been demonstrated that NF-κB cannot be induced by either TNF or IL-1 in cells deficient for IKK-2 (58, 59). In contrast, IKK-1-deficient cells still show inducible NF-κB, albeit at a slightly reduced level (60, 61). The contribution of IKK-1 to Raf-induced NF-κB activity was less clear from our experiments. A dominant-negative version of IKK-1 did not interfere with Raf-mediated cell transformation, overexpression of wild-type IKK-1 did not activate NF-κB by itself, and no synergism between IKK-1 and Raf could be observed (data not shown). Furthermore, in contrast to the observed strong stimulation of the endogenous IKK-2 activity by PMA, IKK-1 kinase activity was only slightly enhanced (data not shown, and ref. 17).
It had been demonstrated that TNF-α treatment results in a strong stimulation of IKK-2 kinase activity, whereas PMA showed only a marginal effect in vivo (19). This finding can be explained by our observation that these two stimuli induce IKK-2 kinase activity with a different kinetic. Consistent with earlier reports, we see only a weak stimulation of IKK-2 kinase activity after 10 min of PMA induction, whereas TNF-α resulted in a strong stimulation. However, after 30 min of PMA treatment, IKK-2 activity was elevated strongly.
The failure of MEK mutants to interfere with Raf-induced NF-κB activation suggests that MEK is not a component of this Raf complex. It has been shown in the yeast system that all components of the MAPK cascade are organized in an active complex by the Ste5 scaffolding protein (62–64). Therefore, one may speculate that different cellular Raf complexes exist that organize distinct functions of the Raf kinase. Although the kinase function of Raf is required for NF-κB induction, it is not because of a direct phosphorylation of the IKK complex by active Raf. Our results rather show that the action of Raf is indirect. Several lines of evidence indicate that Raf-mediated induction of NF-κB is relayed by MEKK1. First, cotransfection of active Raf with dominant-negative MEKK1 strongly reduced κB-dependent reporter gene activity. Second, cotransfection of MEKK1 and active Raf resulted in synergistic activation of a κB-driven reporter construct. This pathway is reminiscent of the mode of activation of NF-κB by Cot/Tpl-2, another member of the MAP(3)K family. Cot-mediated induction of NF-κB is also indirect and involves interaction with and phosphorylation of NIK (65). Interestingly, Cot/Tpl-2 appears to be able to induce NF-κB function by two independent modes, because, in addition to stimulating IKK-activity, Cot/Tpl-2 was also shown to directly interact with the p105/NF-κB1 precursor and induce its degradation (65). As p105 also functions as an inhibitor of NF-κB activity (66), its degradation leads to NF-κB activation.
Acknowledgments
We thank R. Janknecht, M. Karin, D. Wallach, P. Cohen, S. Cowley, and N. Ahn for Gal-Elk, MEKK1 (wild type and dominant negative), NIK (wild type and dominant negative), Raf-BXB-DD, MEK-S217A, and MEK-K97M expression vectors; J. Li for the recombinant IKK-1 and IKK-2 proteins; G. Adolf (Boehringer Ingelheim, Vienna) for human TNF-α; H. Wecklein, R. Metz, and P. Lorenz for excellent technical assistance; and B. Kistler and R. Schreck for very helpful comments on the manuscript. This work was supported by grants to T.W. from the Deutsche Forschungs-Gemeinschaft (DFG Wi789/2-2), the Sander Stiftung (97.031.1), and the Fonds der Chemischen Industrie, a DFG grant to U.R.R. (DFG SFB 176), and a DFG grant (DFG We2023/2-1) to C.K.W.
Abbreviations
- IKK
IκB-kinase
- PMA
phorbol 12-myristate 13-acetate
- NIK
NF-κB-inducing kinase
- HA
hemagglutinin
- GST
glutathione S-transferase
- TNF
tumor necrosis factor
- ERK
extracellular signal-regulated kinase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080583397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080583397
References
- 1.Baldwin A S. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Schreck R, Kistler B, Wirth T. In: Transcription Factors in Eukaryotes. Papavassiliou A G, editor. Austin, TX: R. G. Landes; 1997. pp. 153–188. [Google Scholar]
- 3.Attar R M, Caamano J, Carrasco D, Iotsova V, Ishikawa H, Ryseck R-P, Weih F, Bravo R. Semin Cancer Biol. 1997;8:93–101. doi: 10.1006/scbi.1997.0060. [DOI] [PubMed] [Google Scholar]
- 4.Guttridge D C, Albanese C, Reuther J Y, Pestell R G, Baldwin A S., Jr Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karin M. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 7.Zandi E, Karin M. Mol Cell Biol. 1999;19:4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foo S Y, Nolan G P. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- 9.Whiteside S T, Israel A. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 10.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. Nature (London) 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi M, Rothe M, Goeddel D V. J Biol Chem. 1996;271:19935–19942. doi: 10.1074/jbc.271.33.19935. [DOI] [PubMed] [Google Scholar]
- 12.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. Nature (London) 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 13.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 14.Yeh W-C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, et al. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 15.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 16.Kanakaraj P, Schafer P H, Cavender D, Wu Y, Ngo K, Grealish P F, Wadsworth S A, Peterson P A, Stierkierka J J, Harris C A, Fung-Leung W-P. J Exp Med. 1998;187:2073–2079. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. Nature (London) 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 18.Régnier C, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 19.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 20.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J W, Young D B, Barbosa M, Mann M, Manning A, Rao A. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 21.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 22.Zandi E, Chen Y, Karin M. Science. 1998;281:360–363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 23.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 24.Rothwarf D M, Zandi E, Natoli G, Karin M. Nature (London) 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 25.Cohen L, Henzel W J, Baeuerle P A. Nature (London) 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 26.Ling L, Zhaodan C, Goeddel D V. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumara K. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee F S, Peters R T, Dang L C, Maniatis T. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daum G, Eisenmann-Tappe I, Fries H-W, Troppmair J, Rapp U R. Trends Biochem Sci. 1994;19:474–479. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 30.Bruder J T, Heidecker G, Tan T-H, Weske J C, Derse D, Rapp U R. Nucleic Acids Res. 1993;21:5229–5234. doi: 10.1093/nar/21.22.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finco T S, Baldwin A S. J Biol Chem. 1993;268:17676–17679. [PubMed] [Google Scholar]
- 32.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 33.Kerkhoff E, Rapp U R. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flory E, Weber C K, Chen P, Hoffmeyer A, Jassoy C, Rapp U R. J Virol. 1998;72:2788–2794. doi: 10.1128/jvi.72.4.2788-2794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bender K, Gottlicher M, Whiteside S, Rahmsdorf H J, Herrlich P. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowley S, Paterson H, Kemp P, Marshall C J. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 37.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 38.Kirillov A, Kistler B, Mostoslavsky R, Cedar H, Wirth T, Bergman Y. Nat Genet. 1996;13:435–441. doi: 10.1038/ng0895-435. [DOI] [PubMed] [Google Scholar]
- 39.Annweiler A, Zwilling S, Wirth T. Nucleic Acids Res. 1994;22:4250–4258. doi: 10.1093/nar/22.20.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janknecht R, Ernst W H, Pingoud V, Nordheim A. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumann B, Kistler B, Kirillov A, Bergman Y, Wirth T. J Biol Chem. 1998;273:11448–11455. doi: 10.1074/jbc.273.19.11448. [DOI] [PubMed] [Google Scholar]
- 42.Troppmair J, Bruder J T, Munoz H, Lloyd P A, Kyriakis J, Banerjee P, Avruch J, Rapp U R. J Biol Chem. 1994;269:7030–7035. [PubMed] [Google Scholar]
- 43.Daub M, Jockel J, Quack T, Weber C K, Schmitz F, Rapp U R, Wittinghofer A, Block C. Mol Cell Biol. 1998;18:6698–6710. doi: 10.1128/mcb.18.11.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmeyer A, Avots A, Flory E, Weber C K, Serfling E, Rapp U R. J Biol Chem. 1998;273:10112–9. doi: 10.1074/jbc.273.17.10112. [DOI] [PubMed] [Google Scholar]
- 45.Lernbecher T, Müller U, Wirth T. Nature (London) 1993;365:767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- 46.Pfisterer P, Annweiler A, Ullmer C, Corcoran L, Wirth T. EMBO J. 1994;13:1654–1663. doi: 10.1002/j.1460-2075.1994.tb06429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Betsholtz C, Johnsson A, Heldin C H, Westermark B. Proc Natl Acad Sci USA. 1986;83:6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer M, Sachsenmaier C, Herrlich P, Rahmsdorf H J. J Biol Chem. 1993;268:6734–6741. [PubMed] [Google Scholar]
- 49.Troppmair J, Hartkamp J, Rapp U R. Oncogene. 1998;17:685–690. doi: 10.1038/sj.onc.1201981. [DOI] [PubMed] [Google Scholar]
- 50.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marais R, Wynne J, Treisman R. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 52.Hill C S, Marais R, John S, Wynne J, Dalton S, Treisman R. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Peet G W, Pullen S S, Schembri-King J, Warren T C, Marcu K B, Kehry M R, Barton R, Jakes S. J Biol Chem. 1998;273:30736–30741. doi: 10.1074/jbc.273.46.30736. [DOI] [PubMed] [Google Scholar]
- 54.Finco T S, Westwick J K, Norris J L, Beg A A, Der C J, Baldwin A S., Jr J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 55.Mayo M W, Wang C-Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A S., Jr Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 56.Schouten G J, Vertegaal A C O, Whiteside S T, Israel A, Toebes M, Dorsman J C, van der Eb A J, Zantema A. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghoda L, Lin X, Greene W C. J Biol Chem. 1997;272:21281–21288. doi: 10.1074/jbc.272.34.21281. [DOI] [PubMed] [Google Scholar]
- 58.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 59.Li Z-W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 61.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 62.Choi K Y, Satterberg B, Lyons D M, Elion E A. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 63.Kranz J E, Satterberg B, Elion E A. Genes Dev. 1994;8:313–327. doi: 10.1101/gad.8.3.313. [DOI] [PubMed] [Google Scholar]
- 64.Marcus S, Polverino A, Barr M, Wigler M. Proc Natl Acad Sci USA. 1994;91:7762–7766. doi: 10.1073/pnas.91.16.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin X, Cunningham E T, Mu Y, Geleziunas R, Greene W C. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- 66.Belich M P, Salmeron A, Johnston L H, Ley S C. Nature (London) 1999;397:363–368. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]