Abstract
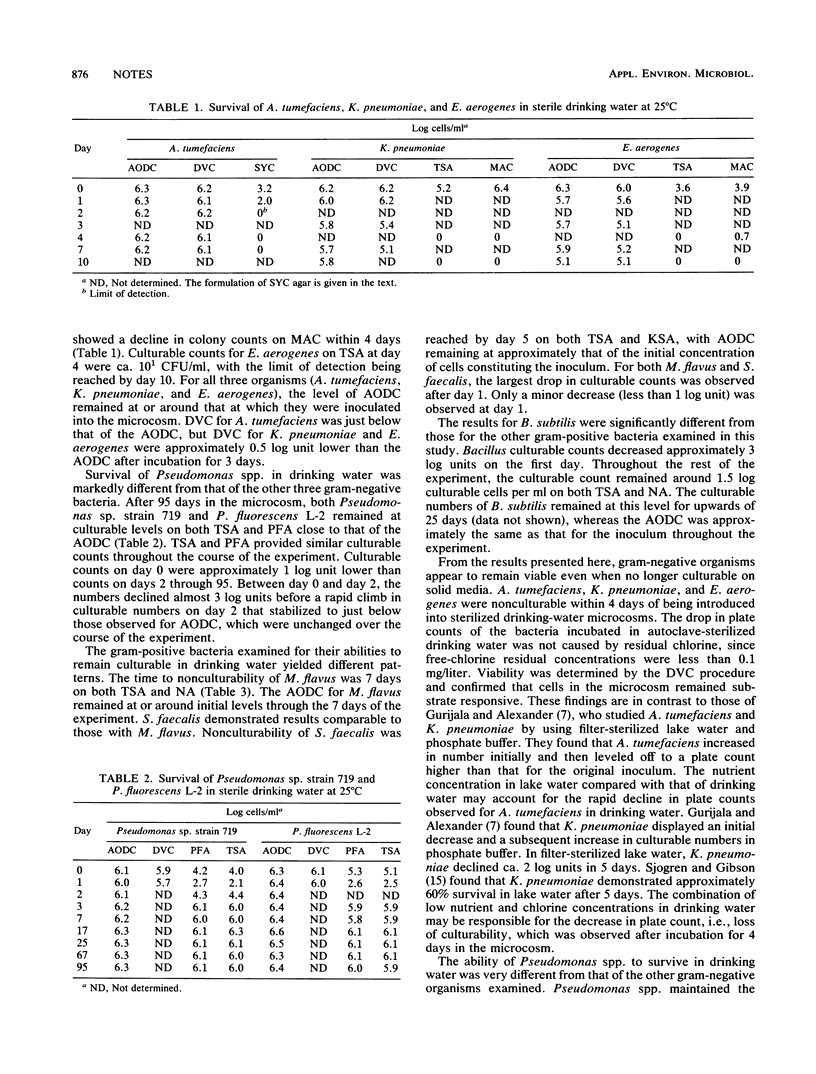
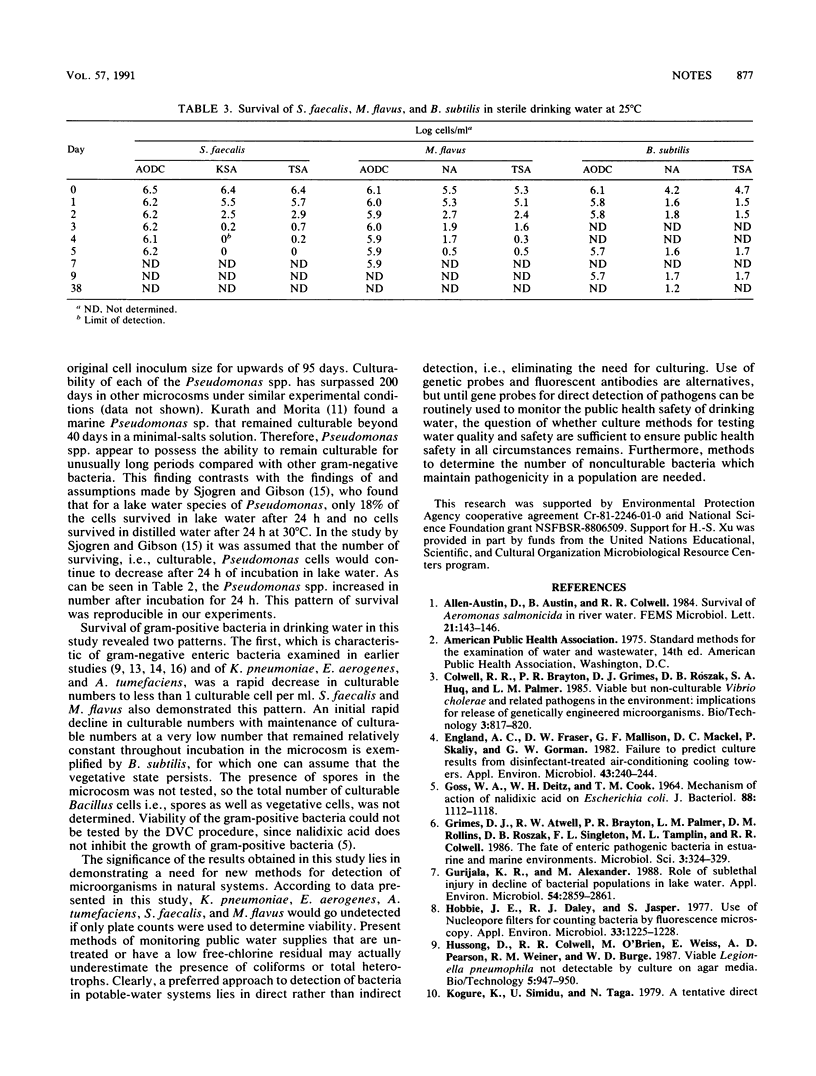
Klebsiella pneumoniae, Enterobacter aerogenes, Agrobacterium tumefaciens, Streptococcus faecalis, Micrococcus flavus, Bacillus subtilis, and Pseudomonas strains L2 and 719 were tested for the ability to grow and maintain viability in drinking water. Microcosms were employed in the study to monitor growth and survival by plate counts, acridine orange direct counts (AODC), and direct viable counts (DVC). Plate counts dropped below the detection limit within 7 days for all strains except those of Bacillus and Pseudomonas. In all cases, the AODC did not change. The DVC also did not change except that the DVC, on average, were ca. 10-fold lower than the AODC.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- England A. C., 3rd, Fraser D. W., Mallison G. F., Mackel D. C., Skaliy P., Gorman G. W. Failure of Legionella pneumophila sensitivities to predict culture results from disinfectant-treated air-conditioning cooling towers. Appl Environ Microbiol. 1982 Jan;43(1):240–244. doi: 10.1128/aem.43.1.240-244.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. J., Atwell R. W., Brayton P. R., Palmer L. M., Rollins D. M., Roszak D. B., Singleton F. L., Tamplin M. L., Colwell R. R. The fate of enteric pathogenic bacteria in estuarine and marine environments. Microbiol Sci. 1986 Nov;3(11):324–329. [PubMed] [Google Scholar]
- Gurijala K. R., Alexander M. Role of sublethal injury in decline of bacterial populations in lake water. Appl Environ Microbiol. 1988 Nov;54(11):2859–2861. doi: 10.1128/aem.54.11.2859-2861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Sjogren R. E., Gibson M. J. Bacterial survival in a dilute environment. Appl Environ Microbiol. 1981 Jun;41(6):1331–1336. doi: 10.1128/aem.41.6.1331-1336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



