Abstract
Cloretazine (VNP40101M) is a newly synthesized alkylating agent belonging to a novel class of alkylating agents called 1,2-bis(sulfonyl)hydrazines. Agents that belong to this class do not produce vinylating and chloroethylating species, and hence this class of alkylating agents is thought to have minimal systemic toxicity. Cloretazine produces two short-lived active species: 1,2-bis(methylsulfonyl)-1-(2-chloroethyl) hydrazine (a chloroethylating species) and a thiophilic carbamoylating methylisocyanate species. The chloroethylating species preferentially produces lesions at the O6 position of guanine. The methylisocyanate species may inhibit O6-alkylguanine-DNA alkyltransferase, an important mechanism of resistance against alkylating agents. The purpose of this study was to determine the efficacy and tolerability of Cloretazine in patients with recurrent glioblastoma multiforme. The basis for the determination of efficacy was the proportion of patients alive without evidence of disease progression six months after initiation of treatment. Patients with recurrent glioblastoma multiforme received Cloretazine (300 mg/m2) intravenously every six weeks. Radiographic response, survival data, and toxicity were assessed. Thirty-two patients were enrolled. Median age was 56 years; 24 patients (75%) were men. At six months, two patients were alive and progression free, so the six-month progression-free survival (PFS) was 6%. The median PFS was 6.3 weeks. There were no objective radiographic responses. Twelve patients had stable disease for at least one cycle, but only two patients received more than three cycles. Nine patients experienced grade 4 thrombocytopenia and three patients experienced grade 4 neutropenia. Cloretazine administered every six weeks was relatively well tolerated, although this schedule has insignificant activity for patients with recurrent glioblastoma multiforme
Keywords: Cloretazine, glioblastoma multiforme
Despite decades of intense basic and clinical research, the median survival for patients with glioblastoma multiforme remains 8–12 months. Therapy consists of surgical debulking followed by radiation and, for most patients, adjuvant chemotherapy (Fine, 1994; Shapiro, 1986). Several small studies suggest a benefit from chemotherapy (Eagan and Scott, 1983; Garrett et al., 1978; Solero et al., 1979). Many of the studies have included nitrosoureas (such as carmustine or lomustine) or temozolomide and have found a modest benefit (Stupp et al., 2004; Walker et al., 1980). As a result of these findings, alkylating agents remain among the most active agents for the treatment of malignant glioma.
In addition to the development of second-generation alkylating agents, a novel class of alkylating agents has recently been developed, the 1,2-bis(sulfonyl)hydrazines (BSHs).3 First-generation nitrosoureas produce four active species: chloroethylating, hydroxyethylating, carbamoylating, and vinylating. Only the chloroethylating and carbamoylating species are associated with the efficacy of these agents, whereas the vinylating and hydroxyethylating species are associated with the side effects seen (Rice et al., 2005). The most active agent in the class of BSHs is Cloretazine (VNP40101M), which produces two short-lived active species: 1,2-bis(methylsulfonyl)-1-(2-chloroethyl) hydrazine (terminal half-life = 30 s at physiologic state), a chloroethylating species that preferentially alkylates the O6 position of guanine; and a thiophilic carbamoylating methylisocyanate species that seems to inhibit O6-alkylguanine-DNA alkyltransferase (AGT), the enzyme that repairs the O6-guanine lesion (Penketh et al., 2000, 2003). AGT activity is an important mechanism of resistance in malignant primary brain tumors (Wiestler et al., 1984).
Two phase I studies have evaluated the toxicity of Cloretazine in patients with advanced solid tumors; a third phase I study evaluated toxicity in patients with hematologic malignancies. In the first study, Cloretazine was given as a one-time 15-min infusion every four to six weeks over 13 dose levels. Twenty-six patients were evaluated, and the maximum tolerated dose for the treatment of solid tumors was approximately 305 mg/m2. The second phase I study for patients with advanced solid tumors is ongoing and is intended to evaluate the toxicity of Cloretazine given as a weekly infusion for three weeks with a one-week rest on a four-week cycle. A third phase I trial was conducted in patients with advanced hematologic malignancies. Thirty-one patients were treated with doses ranging from 220 to 708 mg/m2. Two patients, one with myelodysplastic syndrome treated at the dose level of 300 mg/m2 and one with acute myeloid leukemia treated at 600 mg/m2, achieved complete remission. A 708-mg/m2 dose of Cloretazine was associated with minimal nonhematologic toxicity, with the exception of an infusion-related syndrome that included flushing, headache, and hypotension.
On the basis of the phase I trial results demonstrating limited toxicity, some drug activity in patients with refractory solid tumors or hematologic malignancies, and a lack of significant drug interactions, we performed the first phase II trial of Cloretazine for adult patients with recurrent glioblastoma multiforme.
Patients and Methods
Eligibility
To be eligible for inclusion, patients were required to have a histologically confirmed diagnosis of recurrent or progressive glioblastoma multiforme with no more than three prior recurrences. Histology was reviewed at the study center by a neuropathologist and graded according to the four-tiered WHO system. Patients were required to have measurable recurrent or residual disease and to have recovered fully from prior chemotherapy or radiotherapy.
Patients had to be at least 18 years of age, have a KPS greater than 60%, and have been on a stable dose of corticosteroids for at least one week. Laboratory criteria included hematocrit > 29%, ANC > 1500/μl, platelets > 125,000 cells/μl, creatinine < 1.5 mg/dl, serum glutamic-oxaloacetic transaminase and bilirubin < 1.5 times the upper limits of normal, and diffusion capacity ⩾ 60% of predicted. Exclusion criteria included pregnancy or breast-feeding, comedication that might interfere with study results (e.g., immunosuppressive agents other than corticosteroids), and active infection. All patients were required to understand and sign a written informed consent document approved by the Duke University institutional review board.
Treatment Plan and Dose Modifications
Cloretazine was supplied by Vion Pharmaceuticals (New Haven, Conn.) as a clear, colorless, slightly viscous, sterile, nonaqueous solution for intravenous administration in 10-ml vials containing 100 mg of Cloretazine, 3 ml of anhydrous ethanol, 7 ml of polyethylene glycol 300, and 6 ml of citric acid. Cloretazine was stored under refrigeration, at 2°–8°C (36°–46°F). For patient administration, Cloretazine was diluted in 5% dextrose injection USP to a concentration of 4 mg/ml, for a total volume of 250 ml. The total infusion volume was infused over 15 min.
Cloretazine was administered to all patients at an initial dose of 300 mg/m2 once every six weeks. Therapy was continued until disease progression, grade 4 nonhematologic toxicity, grade 4 hematologic toxicity lasting longer than seven days given the increased risk of prolonged neutropenia or thrombocytopenia, stable disease for one year, complete response for one year, or significant clinical decline. Toxicity was graded using the NCI Common Terminology Criteria for Adverse Events, version 3.0.
A dose reduction of 50 mg/m2 was instituted for any patient with grade 3 nonhematopoietic or grade 4 hematologic toxicity. Two dose reductions were permitted, and new cycles of treatment could be delayed up to four weeks. Patients with grade 4 nonhematologic toxicity were removed from the study. Patients with hematologic toxicity could receive hematopoietic growth factor support at the discretion of the treating physician. Infusion-related reactions, including hypotension, facial flushing, nausea, dizziness, headache, vomiting, and leg cramps, were treated with antihistamines.
Measurement of Effect
The efficacy of Cloretazine was assessed by measuring progression by using MRI, as determined by the Macdonald criteria (Macdonald et al., 1990). Patients underwent a baseline MRI scan before treatment and reevaluation every six weeks with additional MRI scans. The baseline and follow-up scans for each patient were centrally reviewed to determine overall radiographic response. Clinical diagnosis of progressive disease was determined by progressive clinical decline attributed to the progression of tumor, consistent with the Macdonald criteria (Macdonald et al., 1990).
Statistical Considerations
The primary objective of this single-stage study was to evaluate the six-month progression-free survival (PFS) of patients that received an infusion of Cloretazine every six weeks. PFS was defined as the time between initiation of treatment and the first occurrence of disease progression or recurrence, or death. Patients were censored at the time of last follow-up. The product limit estimator of Kaplan and Meier was used to graphically summarize PFS. Yung et al. (2000) reported a six-month PFS rate of 21% (95% CI, 13%–29%) among patients with first-relapse glioblastoma multiforme who were treated with temozolomide. This study was intended to differentiate between 5% and 20% rates of six-month PFS. Statistically, the hypothesis tested was H0: P < 0.05 versus H1: P > 0.2, where P is the proportion of patients who lived six or more months without disease progression. The number of patients required was 32, based on a two-tailed test with alpha = 0.05 and power = 0.95.
Results
Patients and Eligibility
Thirty-two patients were enrolled between May 2004 and September 2004 (Table 1). The accrual goal was met. The median age of the patients was 56 years, with a range of 33–79 years. Response and toxicity could be assessed in all patients. At the time of their original diagnoses, patients had undergone stereotactic biopsy (n = 7), subtotal resection (n = 16), or gross total resection (n = 9). Three patients had received carmustine-impregnated wafers at the time of surgery. All of the patients received radiation therapy. All but one patient had received prior chemotherapy (up to 19 cycles, with a median of four cycles). The patients received a variety of prior chemotherapeutic agents, including nitrosoureas (n = 6) and temozolomide (n = 31). Seventeen patients were enrolled at their first progression, ten after their second progression, and five after their third progression. Of the 32 patients, 20 progressed to radiation therapy with temozolomide, suggesting a group with poor prognosis.
Table 1.
Characteristics of the 32 adult patients with glioblastoma multiforme
| Characteristic | Number of Patients (%) |
|---|---|
| Gender | |
| Male | 24 (75%) |
| Female | 8 (25%) |
| Initial procedure | |
| Surgery | 32 (100%) |
| Biopsy | 7 (22%) |
| Subtotal resection | 16 (50%) |
| Gross total resection | 9 (28%) |
| Prior treatment | |
| Radiation therapy | 32 (100%) |
| Chemotherapy | 31 (97%) |
| Prior nitrosoureas | 6 (19%) |
| Anticonvulsant | |
| None | 14 (44%) |
| Enzyme-inducing antiepileptic drugs (EIAEDs) | 15 (47%) |
| Non-EIAEDs | 3 (9%) |
| Progression | |
| First | 17 (53%) |
| Second | 10 (31%) |
| Third | 5 (16%) |
Measurement of Therapeutic Effect, Time to Progression, and Survival
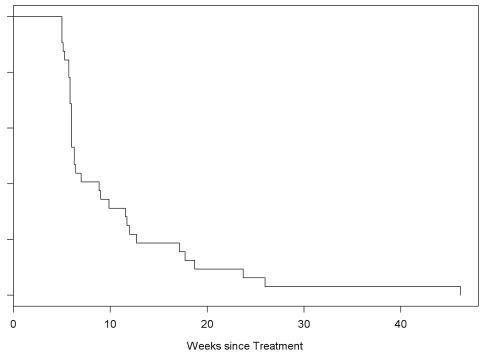
Only two patients had stable disease for six months prior to progression. Twelve had stable disease for at least one cycle, but only two received more than three cycles of treatment. Twenty patients (62.5%) had radiographic progression after one cycle. Of the 32 patients, 31 eventually developed progressive disease. One patient with stable disease after one cycle was removed from the study secondary to hematologic toxicity. The median PFS was 6.3 weeks (95% CI, 6.0–11.6), as shown in Fig. 1. The PFS at six months was 6%. No patient had a complete or partial response.
Fig. 1.
PFS for 32 adult patients with glioblastoma multiforme.
Tolerability and Toxicity
Grade 3 and 4 toxic effects are listed in Table 2. As expected, the most common toxicity was hematologic. There were no differences in the time to progression or in toxicity between the patients who were receiving enzyme-inducing anticonvulsants and those receiving no anticonvulsants or non–enzyme-inducing anticonvulsants.
Table 2.
Numbers of patients (%) with specific toxicities
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| Thrombocytopenia | 6 (19%) | 9 (28%) |
| Neutropenia | 2 (6%) | 3 (9%) |
| Elevated transaminases | 3 (9%) | 0 |
| Cellulitis | 1 (3%) | 0 |
Discussion
In this patient population of adults with glioblastoma multiforme, there were no objective radiographic responses to Cloretazine, although two patients had minor responses. Twenty patients had progressive disease after the first cycle of therapy. Two patients had stable disease for longer than six months. The median PFS was 6.3 weeks (95% CI, 6.0–11.6).
Alkylating agents are important chemotherapeutic drugs used for the treatment of a wide array of solid malignancies, including several regimens for the treatment of CNS neoplasms (Dropcho, 2001). One class of alkylating agents, the nitrosoureas (including lomustine and carmustine), has shown clinical activity in patients with primary CNS neoplasms (Levin et al., 1985). When used alone or in combination with other classes of chemotherapeutic agents, however, the nitrosoureas can be associated with considerable toxicity, including myelosuppression and pneumonitis or pulmonary fibrosis. The nitrosoureas produce several reactive species such as chloroethylating, carbamoylating, vinylating, and hydroxyethylating species. Studies exploring the mechanisms of alkylating agents suggest that the chloroethylating species, which produces DNA cross-linking and results in apoptosis, contributes significantly to the antitumor activity of the drugs. Therefore, alkylating agents that produce a chloroethylating species but lack vinylation or hydroxyethylation properties may have an improved therapeutic–side-effect ratio (Penketh et al., 2000; Rice et al., 2005).
Alkylating agents of the novel BSH class produce a chloroethylating species but do not generate vinylation or hydroxyethylation events. The chloroethylating species preferentially alkylates the O6 position of guanine, although, unlike the nitrosoureas, the BSH derivatives do not attack the N7 position of guanine. The carbamoylating methylisocyanate species produced by Cloretazine seems to increase the antitumor activity of the chloroethylating species. The methylisocyanate is believed to deplete AGT and thus allow the lesion at the O6 position of guanine to persist and to produce robust DNA cross-linking events (Penketh et al., 2000, 2003). Given the acceptable toxicity and novel activity of Cloretazine, we performed this initial phase II study evaluating the efficacy of this agent for the treatment of patients with recurrent glioblastoma multiforme.
Although Cloretazine was well tolerated, it had limited activity in these adults with recurrent glioblastoma multiforme when administered every six weeks. Several factors may account for this observation. AGT is an important mechanism of resistance to alkylating agents found in a host of solid tumors, including malignant brain tumors. We evaluated the efficacy of Cloretazine in the treatment of a panel of human-derived CNS tumor xenografts and noted that overall survival was diminished if the implanted xenograft had high levels of AGT expression (M. A. Badruddoja and H. S. Freedman, unpublished data). Conversely, xenografts with low levels of AGT had the longest survival. Although previous studies have shown that the methylisocyanate species does inhibit AGT, it most likely does so in a limited manner. Cloretazine produces methylation only at the O6 position of guanine and may be only a weak inhibitor of AGT; as a result, this agent may have limited activity against neoplasms with high levels of AGT. Because all but one of our patients had previously progressed to temozolomide, it is likely that this population had elevated levels of AGT. Therefore, since Cloretazine produces only an O6 lesion, tumors from patients enrolled on our study were largely resistant to therapy with Cloretazine. In our study, AGT levels were not evaluated immediately prior to enrollment. In addition, the ability of Cloretazine to deplete AGT may be more robust if Cloretazine is administered more frequently.
Cloretazine seemed to be well tolerated in this patient population but had insignificant activity when given on a schedule of once every six weeks. Given the in vitro activity, Cloretazine should be studied using an alternative schedule, such as weekly administration.
Footnotes
This work was supported by NCI SPORE 1 P20 CA096890.
Abbreviations used are as follows: AGT, O6-alkylguanine-DNA alkyltransferase; BSH, 1,2-bis(sulfonyl)hydrazine; PFS, progression-free survival.
References
- Dropcho E. Novel chemotherapeutic approaches to brain tumors. Hematol Oncol Clin North Am. 2001;15:1027–1052. doi: 10.1016/s0889-8588(05)70266-5. [DOI] [PubMed] [Google Scholar]
- Eagan RT, Scott M. Evaluation of prognostic factors in chemotherapy of recurrent brain tumors. J Clin Oncol. 1983;1:38–44. doi: 10.1200/JCO.1983.1.1.38. [DOI] [PubMed] [Google Scholar]
- Fine HA. The basis for current treatment recommendations for malignant gliomas. J Neurooncol. 1994;20:111–120. doi: 10.1007/BF01052722. [DOI] [PubMed] [Google Scholar]
- Garrett MJ, Hughes HJ, Freedman LS. A comparison of radiotherapy alone with radiotherapy and CCNU in cerebral glioma. Clin Oncol. 1978;4:71–76. [PubMed] [Google Scholar]
- Levin VA, Wara WM, Davis RL, Vestnys P, Resser KJ, Yatsko K, Nutik S, Gutin PH, Wilson CB. Phase III comparison of BCNU and the combination of procarbazine, CCNU, and vincristine administered after radiotherapy with hydroxyurea for malignant gliomas. J Neurosurg. 1985;63:218–223. doi: 10.3171/jns.1985.63.2.0218. [DOI] [PubMed] [Google Scholar]
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- Penketh, P., Shyam, K., Baumann, R., Finch, R., Brent, T., and Sartorelli, A. (2003) In vitro inhibition of O6-alkylguanine-DNA alkyltransferase (AGT) by electrophilic species generated during the decomposition of 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[(methylamion) carbonyl]hydrazine (VNP40101M). 94th Annual Meeting of the American Association for Cancer Research, Toronto, Ontario, Canada, July 11–14.
- Penketh PG, Shyam K, Sartorelli AC. Comparison of DNA lesions produced by tumor-inhibitory 1,2-bis(sulfonyl)hydrazines and chloroethylnitrosoureas. Biochem Pharmacol. 2000;59:283–291. doi: 10.1016/s0006-2952(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Rice KP, Penketh PG, Shyam K, Sartorelli AC. Differential inhibition of cellular glutathione reductase activity by isocyanates generated from the antitumor prodrugs Cloretazine and BCNU. Biochem Pharmacol. 2005;69:1463–1472. doi: 10.1016/j.bcp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Shapiro WR. Therapy of adult malignant brain tumors: What have the clinical trials taught us? Semin Oncol. 1986;13:38–45. [PubMed] [Google Scholar]
- Solero CL, Monfardini S, Brambilla C, Vaghi A, Valagussa P, Morello G, Bonadonna G. Controlled study with BCNU vs. CCNU as adjuvant chemotherapy following surgery plus radiotherapy for glioblastoma multiforme. Cancer Clin Trials. 1979;2:43–48. [PubMed] [Google Scholar]
- Stupp, R., Mason, W., Van Den Bent, M., Weller, M., Fisher, B., Taphoorn, M., Brandes, A., Cairncross, G., Lacombe, D., and Mirimanoff, R. (2004) Concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) for newly diagnosed glioblastoma multiforme (GBM): Conclusive results of a randomized phase III trial by the EORTC brain & RT groups and NCIC clinical trials group. 40th Annual Meeting of the American Society of Clinical Oncology, New Orleans, Louisiana, June 5–8.
- Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, Owens G, Ransohoff J, II, Robertson JT, Shapiro WR, Smith KR, Jr, Wilson CB, Strike TA. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Wiestler O, Kleihues P, Pegg AE. O6-alkylguanine-DNA alkyltransferase activity in human brain and brain tumors. Carcinogenesis. 1984;5:121–124. doi: 10.1093/carcin/5.1.121. [DOI] [PubMed] [Google Scholar]
- Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]