Abstract
Despite multimodal treatment options, the response and survival rates for patients with malignant gliomas remain dismal. Clinical trials with convection-enhanced delivery (CED) have recently opened a new window in neuro-oncology to the direct delivery of chemotherapeutics to the CNS, circumventing the blood-brain barrier and reducing systemic side effects. Our previous CED studies with liposomal chemotherapeutics have shown promising antitumor activity in rodent brain tumor models. In this study, we evaluated a combination of nanoliposomal topotecan (nLs-TPT) and pegylated liposomal doxorubicin (PLD) to enhance efficacy in our brain tumor models, and to establish a CED treatment capable of improving survival from malignant brain tumors. Both liposomal drugs decreased key enzymes involved in tumor cell replication in vitro. Synergistic effects of nLs-TPT and PLD on U87MG cell death were found. The combination displayed excellent efficacy in a CED-based survival study 10 days after tumor cell implantation. Animals in the control group and those in single-agent groups had a median survival of less than 30 days, whereas the combination group experienced a median survival of more than 90 days. We conclude that CED of two liposomal chemotherapeutics (nLs-TPT and PLD) may be an effective treatment option for malignant gliomas.
Keywords: brain tumor, CED, convection-enhanced delivery, glioma, liposome, topotecan
Despite intensive multimodal treatment such as surgical resection, malignant glioma (e.g., glioblastoma multiforme) remains the most difficult neoplasm to treat. Poor penetration of most anticancer drugs across the blood-brain barrier (BBB)4 into the CNS after systemic administration is the one of the major obstacles to improvement in survival for this group of patients. Even with drugs that penetrate the BBB, it is difficult to reach sufficient drug concentrations in brain tumor tissue without causing considerable systemic toxicity (Groothuis, 2000).
Convection-enhanced delivery (CED) is a promising local delivery technique. This technique uses bulk flow to deliver small or large molecules directly to targeted sites in clinically significant volumes of tissue, and it provides wider distribution as compared with simple diffusion techniques (Bobo et al., 1994). CED of therapeutic agents circumvents the BBB, delivering a high concentration of therapeutic agent in the immediate vicinity of the tumor, minimizing systemic exposure, and thus results in a significant shift in the toxicity profile for the drug.
However, because CED distributes therapeutic agents not only to the tumor mass, but also beyond the tumor margin into normal surrounding brain tissue, a strategy for minimizing toxicity to normal brain tissue is needed. Liposomal drugs are promising candidates for local delivery in this regard, because they are inert until the drug is released from the confines of the nanocarrier. Liposomes, microscopic phospholipid nanoparticles with a bilayered membrane structure, are vehicles for administering therapeutic agents, such as drugs and genes, to areas of the body afflicted with cancer. Liposomes have been shown to provide stable encapsulation for various anticancer drugs and have many advantages over the corresponding free drugs for the systemic treatment of cancer (Allen and Martin, 2004; Drummond et al., 1999). One potential advantage of liposome-encapsulated cytotoxic drugs over corresponding unencapsulated agents is prolongation of systemic drug half-life (Allen et al., 1995; Drummond et al., 1999; Gabizon et al., 2003). We have recently developed a novel intraliposomal stabilization strategy for preparing highly stable nanoliposomal drugs (Drummond et al., 2005). In conjunction with CED, it was expected that antineoplastic drugs would be released slowly in the brain, prolonging exposure of brain tumor cells to the drugs, and that this delivery pattern would provide greater efficacy and less toxicity than treatment with free drugs. In fact, CED of liposomal antineoplastic agents demonstrated an excellent selective toxicity against tumor tissue (Noble et al., 2006; Saito et al., 2006a, b). We have also demonstrated that CED of liposomal CPT-11 resulted in no apparent leakage of drug from the CNS to the systemic circulation and produced no signs of systemic side effects (Noble et al., 2006).
Topoisomerase inhibitors have played an important role in cancer chemotherapy. There has therefore been considerable interest in combining topoisomerase I (topo I) inhibitors with topoisomerase II (topo II) inhibitors, because agents that target topo I and topo II exert their principal effects on the two major classes of enzymes involved in regulating DNA topology, which overlap functionally. The feasibility of administering a combination of topo I and topo II inhibitors to patients with advanced solid malignancies has been evaluated in numerous clinical studies (Ando et al., 1997; Hammond et al., 1998; Herben et al., 1997; Penson et al., 2004). In the treatment of patients with brain tumors, both topo I and topo II inhibitors have been found to be promising agents (Gross et al., 2005; Hau et al., 2004; Pipas et al., 2005). We have started to evaluate the combination of nanoliposomal topotecan (nLs-TPT) to inhibit topo I and pegylated (polyethylene glycol–coated) liposomal doxorubicin (PLD) to inhibit topo II, in conjunction with CED in intracranial glioblastoma xenografts. Our previous studies with each liposomal agent alone in the treatment of various intracranial glioblastoma xenografts encouraged us to explore this combination further (Saito et al., 2006a, b; Yamashita et al., 2006). In this study, we used PLD and nLs-TPT to treat U87MG intracranial xenografts. The liposomal drug combination displayed synergy in our in vitro studies and excellent efficacy in large tumors (U87MG, day 10) in our survival studies.
Materials and Methods
Liposome Preparation
Pegylated liposomal doxorubicin (Doxil; Alza Pharmaceuticals, Mountain View, Calif.) was obtained commercially. The commercial PLD solution contained 2 mg/ml of doxorubicin. The PLD was diluted with HEPES (N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]; pH 6.5)-buffered saline (HBS).
Topotecan and nLs-TPT were provided by Hermes Bioscience, Inc. (South San Francisco, Calif.). Topotecan-loaded liposomes were prepared with a modified remote-loading ion gradient and intraliposomal drug stabilization method, as described previously (Saito et al., 2006a). Briefly, after the lipids were dried in a solution of chloroform and methanol (9:1, vol/vol), they were next dried by rotary evaporation and then under vacuum for a minimum of 2 h. The lipids were subsequently resuspended in one volume of ethanol at 60°C. An aqueous solution of triethylammonium sucrose octasulfate (TEA8SOS; 0.65 M TEA) was prepared by cation-exchange chromatography as described previously (Drummond et al., 2005) and was also heated to 60°C. Nine volumes of the TEA8SOS solution were then injected rapidly into the ethanolic lipid solution to form liposomes. The liposomes were sized by extrusion through polycarbonate filters of average size 0.1 μm, which resulted in an average size of 100–120 nm. Unencapsulated TEA8SOS was removed by Sepharose CL-4B size-exclusion chromatography and eluted with water. HEPES and NaCl were added to adjust the final concentrations to 5 mM and 145 mM, respectively. Topotecan was added at a ratio of 350 g of TPT/mol of phospholipid, and loading was initiated by adjusting the pH to 6.5 and incubating the mixture for 30 min at 60°C, followed by quenching on ice for 15 min. Unencapsulated TPT was removed by Sephadex G-75 gel filtration chromatography, eluting with HBS (5 mM HEPES and 145 mM NaCl, pH 6.5), and the drug concentration and the phospholipid concentration of the purified solution were determined spectrophotometrically at 375 nm after dissolution in acidic methanol and by a standard phosphate assay (Bartlett et al., 1959), respectively. The loading efficiency was always greater than 95% for the preparations used in these studies.
DiIC18(3)-DS (DiI-DS) liposomes (control liposomes) were prepared with the fluorescent membrane dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiIC18; Sigma, St. Louis, Mo.) as described previously by Saito et al. (2006a). Briefly, dioleoylphosphatidylcholine (DOPC), cholesterol (molar ratio, 3:2), polyethylene glycol–disteroyl phosphatidylethanolamine (PEG-DSPE; 0.5 mol% of phospholipid), and DiI-DS (0.5 mol%) were codiluted in chloroform and dried in a vacuum by rotary evaporation to form a lipid film. The lipid film was hydrated by shaking it in HBS, followed by six successive cycles of freezing at −80°C and thawing at 37°C. The resulting multilamellar liposomes were extruded through 0.1-μm polycarbonate membrane filters, yielding liposomes of an average diameter of 95.8 nm as determined by dynamic light scattering. The liposome concentration was measured by a standard phosphate assay, and the final liposome concentration was adjusted to 20 mM of phospholipid by diluting the suspension with HBS.
Distribution of Liposomes in Rodent CNS
The role of pegylation in regulating the distribution of liposomes in the brain upon administration by CED appears to be relatively complex. MacKay et al. (2005) have recently observed that liposomes stabilized with the neutral PEG2000-distearoylglycerol lipid displayed a volume of distribution that was 40% greater than observed for similarly constructed nonpegylated liposomes. However, we have recently observed that CED of liposomes prepared with the negatively charged PEG2000-DSPE lipid, similar to the highly pegylated liposomal doxorubicin employed in this study, had a volume of distribution that was nearly identical to nonpegylated liposomes (Saito et al., 2006b). Thus, we expect that the PLD and nLs-TPT coinfused in these studies will have similar distributions in the brain, despite the difference in pegylation of the carrier.
Tumor Cell Line
An established human glioblastoma multiforme cell line, U87MG, was obtained from the Brain Tumor Research Center Tissue Bank at the University of California, San Francisco. Cells were maintained as monolayers in a complete medium that consisted of Eagle’s minimal essential medium supplemented with 10% fetal calf serum and nonessential amino acids. Cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Western Blotting for Topoisomerase I, Topoisomerase II, Cleaved Caspase-3, and α-Tubulin
One day prior to treatment, 4 × 105 cells per well were seeded into six-well multiwells (Corning Inc., Corning, N.Y.). After 24 h of incubation, cells were exposed to DiI-DS liposomes, PLD (5 μg doxorubicin/ml), nLs-TPT (1 μg TPT/ml), or both (PLD at 2.5 μg doxorubicin/ml + topotecan liposomes at 0.5 μg TPT/ml) in complete medium. After a 24-h incubation, cells were collected, and protein was extracted in cell lysis buffer (Cell Signaling Technology, Beverly, Mass.). Equal amounts of protein (10 μg) were separated on a 12% bis-tris gel (Invitrogen, Carlsbad, Calif.) and electroblotted onto an Immobilon-P membrane (Millipore, Bedford, Mass.). The membrane was blocked in 5% skim milk and incubated with primary antibodies against topoisomerase I (1:2000; TopoGEN, Port Orange, Fla.), topoisomerase IIα (1:1000; Chemicon, Calif.), cleaved caspase-3 (1:1000; Cell Signaling Technology), or α-tubulin (1:2000; Santa Cruz Biotechnology, Calif.) overnight at 4°C. Bound antibody was detected with horseradish peroxidase–conjugated secondary antibodies by using ECL Western blotting detection reagents (Amersham, Piscataway, N.J.).
Cell Cycle Analysis
One day prior to treatment, 2 × 105 cells/well were seeded into each well of a six-well plate (Corning Inc.). After a 24-h incubation, cells were exposed to DiI-DS liposomes (control liposomes), PLD (2.5 μg doxorubicin/ml), nLs-TPT (0.5 μg TPT/ml), or both (PLD, 2.5 μg doxorubicin/ml, + topotecan liposomes, 0.5 μg TPT/ml) in complete medium. After 24 h, cells were fixed in 70% ethanol, washed, digested with RNaseA (Sigma), stained with propidium iodide (Sigma), and subjected to flow cytometry in a FACScan machine (Becton-Dickinson, San Jose, Calif.) with 10,000 events per determination. ModFit LT software (Verity Software House, Inc., Topsham, Maine) was used to assess the cell cycle distribution.
MTT Cell Viability Assay
Cells were seeded at 1000 cells per well in 96-well plates (Corning Inc.), allowed to attach for 24 h, and then exposed to DiI-DS liposomes (control liposomes), PLD, nLs-TPT, or both, in complete medium. MTT (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium) reagent was added 48 h after initiation of treatment, and plates were read at an absorbance of 490 nm 3 h later with a Spectra-Max microplate reader (Molecular Device Corporation, Sunnyvale, Calif.). All treatments were performed in triplicate. The background absorbance was determined by incubating media with substrate alone and subtracting the values from wells containing cells only.
Animals and Intracranial Xenograft Technique
Male Sprague-Dawley rats (250 g) were obtained from Charles-River Laboratories (Wilmington, Mass.). Congenitally athymic, male, homozygotic, nude rats (rnu/rnu; 150–200 g) were purchased from the National Cancer Institute (Bethesda, Md.) and housed under aseptic conditions that included filtered air, as well as sterilized food, water, bedding, and cages. All protocols were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco. For the intracranial xenograft tumor model, U87MG cells were harvested by trypsinization, washed once with Hanks’ balanced salt solution without Ca2+ and Mg2+ (HBSS), and resuspended in HBSS for implantation. A cell suspension containing 5 × 105 cells/10 μl of HBSS was implanted into the striatal region of the athymic rat brains. Under deep isoflurane anesthesia, rats were placed in a small-animal stereotactic frame (David Kopf Instruments, Tujunga, Calif.). A sagittal incision was made through the skin to expose the cranium, and a burr hole was made in the skull at 0.5 mm anterior and 3 mm lateral from the bregma with a small dental drill. At a depth of 4.5 mm from the brain surface, 5 μl of cell suspension was injected; 2 min later, another 5 μl was injected at a depth of 4 mm; and 2 min later, the needle was removed and the wound sutured.
Convection-Enhanced Delivery
Throughout this study, free doxorubicin or PLD was delivered in a volume of 20 μl with the CED method described previously (Bankiewicz et al., 2000; Saito et al., 2004). Briefly, the infusion system consisted of a fused-silica needle cannula that was connected to a loading line (containing liposomes) and an oil-infusion line. A 1-ml syringe (filled with oil) was mounted onto a microinfusion pump (BeeHive; Bioanalytical Systems, West Lafayette, Ind.) that regulated the flow of fluid through the system. By referring to tumor injection site coordinates, the step-design cannula was mounted onto stereotactic holders and guided to the targeted region of the brain through burr holes made in the skull (see the previous section for details). The following ascending infusion rates were applied to achieve the 20-μl infusion: 0.2 μl/min (15 min) + 0.5 μl/min (10 min) + 0.8 μl/min (15 min). The step-design cannula used for all CED procedures in this study was reflux free and backflow free at infusion rates up to 50 μl/min (Krauze et al., 2005a).
Evaluation of Toxicity
Three normal Sprague-Dawley rats were evaluated for potential local toxicity after CED-mediated coinfusion of PLD and nLs-TPT. CED was performed as described in the previous section. Rats were monitored daily for general health (alertness, grooming, feeding, excreta, skin, fur, mucous membrane condition, ambulation, breathing, and posture). Animal weights were reported weekly. Both liposomal drugs were used at half the maximum tolerated dose (MTD) that was determined in previous studies (Saito et al., 2006b; Yamashita et al., 2006). Sixty days after CED of 20 μl of PLD (0.1 mg/ml) and 20 μl of nLs-TPT (0.25 mg/ml) into the striatum, rats were euthanized and their brains fixed in 4% formaldehyde. Fixed brain tissue was subjected to paraffin sectioning (5 μm) and stained with hematoxylin and eosin (H&E).
Combination Therapy Against the U87MG Intracranial Xenograft Model
Thirty-two rats, implanted with U87MG tumor cells, were randomly divided into four CED treatment groups of eight rats each: (1) a control group treated with DiI-DS fluorescent liposomes, (2) a group treated with PLD (0.1 mg/ml doxorubicin), (3) a group treated with nLs-TPT (0.25 mg/ml), and (4) a group receiving a combination treatment of PLD (0.1 mg/ml) together with nLs-TPT (0.25 mg/ml) (n = 8). Ten days after tumor cell implantation, a CED of 20 μl of the specified drug was performed for each group. Rats were monitored daily for survival and general health (alertness, grooming, feeding, excreta, skin, fur, mucous membrane conditions, ambulation, breathing, and posture). Animal weights were reported weekly. The study was terminated 90 days after tumor implantation, surviving animals were euthanized, and their brains were stained with H&E. An additional three rats were implanted with U87MG tumor cells and euthanized 10 days after implantation to estimate the tumor size on the treatment day used in this study.
Statistical Analysis
Results for the survival studies were expressed as a Kaplan-Meier curve. Survival of the treatment groups was compared with a log-rank test and median survival.
Results
Expression of Topoisomerase I and Topoisomerase IIα in U87MG Cells
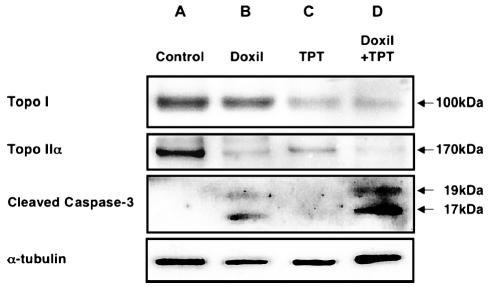
Both topo I and topo II were highly expressed in U87MG cells (Fig. 1A). After treatment with nLs-TPT, depletion of topo I expression was observed (Fig. 1C). A similar decrease in topo II was seen (Fig. 1C), confirming that no compensatory increase in topo II occurred. Treatment with PLD resulted in a reduction in topo II expression, but there was no significant effect on the expression of topo I (Fig. 1B). After simultaneous exposure to PLD and nLs-TPT, a significant reduction in both topoisomerase isoforms was observed (Fig. 1D).
Fig. 1.
Western blotting for topo I, topo IIα, and cleaved caspase-3. U87MG cells were incubated for 24 h with PLD (5 μg/ml), nLs-TPT (1 μg/ml), or a combination of PLD (2.5 μg/ml) and nLs-TPT (0.5 μg/ml). Western blotting was used for detection of topo I, topo IIα, cleaved caspase-3, and α-tubulin. A. The untreated control cells(U87MG) show high levels of topo I and topo II α expression. B. Cells incubated with PLD (Doxil) show downregulation of topo IIα, upregulation of cleaved caspase-3, and similar levels of topo I when compared with control cells. C. Cells incubated with nLs-TPT show downregulation of topo I and topo IIα and no signal for cleaved caspase-3. D. The combination of PLD and nLs-TPT shows downregulation of topo I and topo IIα and increased levels of cleaved caspase-3.
The molecular events occurring during synergistic induction of cell death by PLD and nLs-TPT were characterized in U87MG cells at approximate EC50 concentrations (concentrations that were effective in inducing death in 50% of the U87MG cells). Western blots for cleaved caspase-3 detected the activation of caspase-3 in U87MG cells treated with PLD (5 μg/ml) (Fig. 1B), or at considerably higher levels upon coexposure to PLD (2.5 μg/ml) and nLs-TPT (1 μg/ml) (Fig. 1D). After treatment with the combination of PLD and nLs-TPT, caspase-3 activation increased considerably, even though both liposomal drugs were used at approximate EC25 concentration, half of the dose employed in the single-drug treatment groups.
Synergistic Cytotoxic Effects of PLD and Topotecan Liposome In Vitro
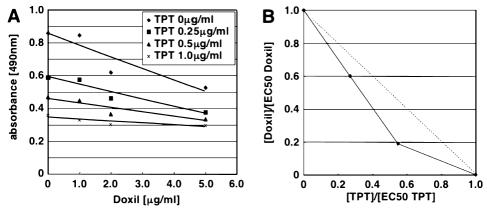
Synergy, as determined by isobologram analysis (Berenbaum, 1981), was observed between the two agents (Fig. 2). EC50 values (median effect doses) calculated from this experiment were 6.08 μg/ml for PLD and 0.91 μg/ml for topotecan liposome and were plotted on a graph to show synergy (values under the dashed line Fig. 2B).
Fig. 2.
Synergistic induction of cell death by PLD and nLs-TPT in U87MG glioma cells. A. U87MG cells were treated for 48 h with increasing nLs-TPT concentrations (0–1 μg/ml) and 24 h with increasing PLD concentrations (0–5 μg/ml). B. Synergy between the two agents is expressed in an isobologram.
Cell Cycle Distributions In Vitro
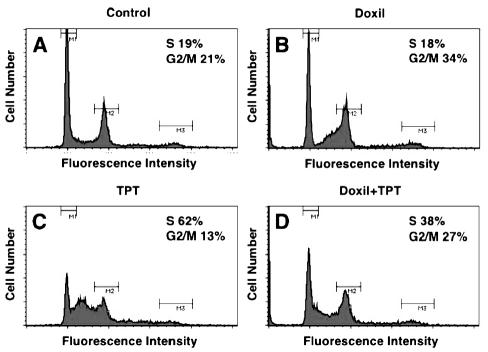
Although activation of caspase-3 was not observed within 24 h after treatment with nLs-TPT in U87MG cells, prolonged exposure of these cells to nLs-TPT resulted in marked S-phase accumulation (62% with topotecan liposomes vs. 19% with control liposomes; Fig. 3C and Fig. 3A, respectively). In contrast, cells treated with PLD, a G2/M-active antineoplastic drug, underwent G2 arrest, with the percentage of cells in G2/M increasing from 21% prior to PLD treatment to 34% 24 h later (Fig. 3B). At 24 h after combination treatment with PLD and nLs-TPT, both S-phase accumulation and G2 arrest were observed in U87MG cells (Fig. 3D).
Fig. 3.
Cell cycle profiles of U87MG cells examined by flow cytometry. U87MG cells were harvested for analysis and exposed to A. DiI-DS fluorescent liposomes (control), B. PLD (2.5 μg/ml), C. topotecan liposomes (TPT) (0.5 μg/ml), or D. a combination of PLD (2.5 μg/ml) and TPT (0.5 μg/ml) for 24 h.
Toxicity of Combination Treatment in Normal Rat Brain
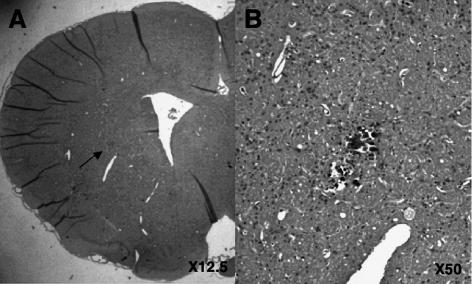
The rats receiving the combination treatment (CED infusion with 0.1 mg/ml of doxorubicin as PLD and 0.25 mg/ml of nLs-TPT) survived without any neurological symptoms, and the tissue damage was negligible (Fig. 4). The MTD for each therapeutic was determined in previous studies, that is, 0.2 mg/ml for doxorubicin as PLD and 0.5 mg/ml for nLs-TPT. No increase in tissue damage was observed in comparison with similar data from previous studies (Saito et al., 2006a; Yamashita et al., 2006).
Fig. 4.
Toxicity evaluation. Sixty days after CED infusion of 0.1 mg/ml of doxorubicin as PLD and 0.25 mg/ml of topotecan as liposomal drug into the striatum of intact Sprague-Dawley rats (n = 3), rats were euthanized, and 5-μm paraffin sections of their brains were obtained. A. Representative H&E sections from a single rat are shown. B. Only a small region of inflammation was detected just adjacent to the needle tract.
Combined Effect of PLD and nLs-TPT in U87MG Brain Tumor Xenografts In Vivo
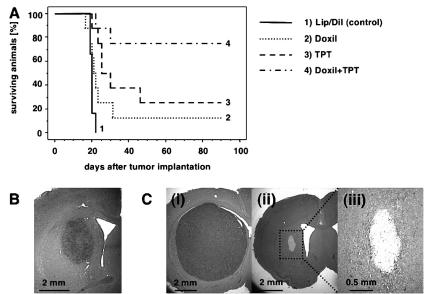
Rats in the control group, which received liposomes tagged with the fluorescent dye DiI-DS, were all euthanized 19 to 22 days after tumor cell implantation because of neurological symptoms indicative of tumor progression. The median survival (MS) for this group was 20 days (Fig. 5A-1). One of eight rats that received 0.1 mg/ml PLD by CED survived until termination of the study. However, neurological symptoms caused by large tumor formations were observed in the seven other rats, requiring euthanasia at 16 to 31 days after tumor cell implantation. No significant improvement in survival was noted in this treatment group (P = 0.0789), with an MS of 21.5 days (Fig. 5A-2). Two of eight rats that received treatment at 0.25 mg/ml nLs-TPT by CED survived until termination of the study. Because of neurological symptoms indicating tumor progression, the six other rats in this group had to be euthanized 20 to 46 days after tumor cell implantation. In contrast with treatment with PLD, a significant survival benefit for nLs-TPT was found (P = 0.002), with an MS for this group of 27.5 days (Fig. 5A-3). However, rats in the group that received the combination treatment of PLD and nLs-TPT demonstrated a significant improvement in survival (P = 0.0004), surviving significantly longer than the rats in the single-agent therapy groups, with six of eight rats surviving until termination of the study (MS > 90 days) (Fig. 5A-4). To estimate tumor size at time of treatment, three additional rats from the control group were euthanized on day 10 after tumor implantation (Fig. 5B).
Fig. 5.
Survival study. A. Chemotherapy with a combination of PLD and topotecan liposomes prolongs the survival of U87MG brain tumor xenografts. Survival of treated animals was observed for 90 days and is expressed as a Kaplan-Meier curve. Eight rats for each group were used in the experimental design of the survival study. B. U87MG xenograft size on day 10 after tumor cell implantation after treatment with DiI liposomes (control) (n = 3, H&E staining). In histological findings at necropsy, H&E staining of the brains of rats that failed to survive the observation period revealed large tumor formation C. (i), whereas in the brains of rats that received combination therapy and survived the observation period, fibrous scar tissues were detected C. (ii), (iii).
Discussion
Clinical trials to evaluate the combination of topo I and topo II inhibitors via intravenous administration in patients with solid neoplasms have demonstrated substantial toxicity (Ando et al., 1997; Hammond et al., 1998; Herben et al., 1997). Because the defined MTD was frequently lower than the typical dose level for the respective individual agent, the efficacy with systemic treatment was often limited by hematologic toxicities (Penson et al., 2004). For patients with brain tumors, systemic delivery of therapeutics is usually associated with systemic side effects while achieving marginal therapeutic concentrations in the CNS; thus the efficacy of systemic treatment is also limited. In an effort to improve drug delivery to the brain and to reduce systemic side effects caused by antineoplastic agents, recent studies have demonstrated the advantages of CED (Saito et al., 2004). Chemotherapeutic agents delivered locally by CED have produced favorable therapeutic outcomes (Bruce et al., 2000; Degen et al., 2003; Kaiser et al., 2000). However, highly cytotoxic agents with extensive distribution in the CNS have resulted in brain damage (Kaiser et al., 2000). Hence, good candidates for CED administration into brain tumors would ideally be the agents that show the highest possible therapeutic index against tumor cells over healthy neuronal cells. Liposomal drug delivery offers the potential for avoiding the high peak concentrations of bioavailable drug that are so often associated with pronounced toxicity (Noble et al., 2006).
In preclinical studies, topo I and topo II inhibitors have already been combined in many schedules and models. Topo I mRNA and protein levels in the tumor decreased, whereas topo II mRNA and protein levels rose after treatment with topo I inhibitor. The reverse effect, a fall in topo II and concurrent rise in topo I levels with topo II–active drug treatment, was also observed. The conclusion was that topo I and II agents can be combined to produce greater than additive tumor cytotoxicity without increase in host toxicity (Eder et al., 1998). In addition, initial treatment with topotecan was associated with increasing responsiveness of the xenografts to subsequent doses of doxorubicin (Kim et al., 1992). On the basis of these observations, we designed this study to determine whether a synergistic effect in vitro and in the U87MG intracranial rodent xenograft model in vivo could be observed.
It is generally accepted that the sensitivity of proliferating tumor cells to DNA-damaging agents is higher than that of quiescent tumor cells. Topo I inhibition increases the number of covalent topo I–DNA complexes within cells, and the interaction of these complexes with replication forks results in the formation of double-strand DNA breaks (Kaufmann et al., 1996; Rowinsky and Kaufmann, 1997). These DNA lesions are thought to be responsible for topotecan-induced cell death. This mechanism accounts for the maximum cytotoxicity of topotecan observed during the S phase of the cell cycle (Sinha, 1995; Taron et al., 2000). It has already been reported that prolonged exposure to low doses of topo I inhibitors, such as topotecan, results in an increase in G2-phase and/or S-phase cells (Cliby et al., 2002; Kim et al., 1992). S-phase cells increased in our U87MG cells after treatment with a novel, highly stable nanoliposomal topotecan. Furthermore, it was found that after topotecan administration, the subsequent administration of DNA-damaging antineoplastic drugs, at the interval of maximal topotecan-induced S-phase cell cycle arrest, could increase DNA damage, inhibiting DNA repair and G2/M transit, thus synergistically increasing cytotoxicity (Taron et al., 2000). It was expected that doxorubicin would be released slowly from the liposomes, to prolong the exposure of brain tumor cells that showed S-phase accumulation induced by topotecan liposomes, and that this delivery pattern would thereby provide a synergistic increase in efficacy (Allen and Martin, 2004). Additionally, we have shown previously that locally administered topotecan liposomes work as a drug source for effective antiangiogenic chemotherapy in malignant glioma xenografted models (Saito et al., 2006a). It was also reported that the breakdown of tumor vasculature induced by doxorubicin-containing liposomes might arise both from the vascular accumulation of liposomes and from cytotoxic effects on tumor vascular endothelial cells (Zhou et al., 2002).
In our previous study (Saito et al., 2006a), CED of nLs-TPT eradicated tumors and significantly enhanced survival of tumor-bearing rats, in comparison to similar animals that received only systemic administration of topotecan, a result that encouraged further exploration. On the other hand, the limited efficacy observed in a late treatment of a U87MG xenograft model clearly showed the necessity not only of highly selective toxicity, but also of improved cytotoxic activity toward tumor cells in vivo in more developed tumors (Saito et al., 2006a). In this study, by means of CED of a combination of PLD and nLs-TPT, we significantly prolonged the survival of tumor-bearing rats without increasing toxicity in the normal brain, even though the start of treatment was delayed as compared with the earlier study.
Our main aim in this study was to develop an effective treatment for the eradication of large intracranial U87MG tumor xenografts (day 10). As malignant human brain tumors are usually detected at an advanced stage, our study also focused on advanced tumor xenografts with an established vasculature. Rats receiving treatment on day 10 after U87MG implantation were approximately halfway through their average life expectancy after tumor inoculation. The combination of PLD and nLs-TPT showed impressive effects in vitro, and CED of this chemotherapeutic combination significantly improved therapeutic outcome in a large human U87MG glioma intracranial xenograft model.
We propose that this CED approach be used in future clinical studies. It seems likely that this new combination therapy may offer a new and potentially effective chemotherapeutic regimen in glioma therapy. As demonstrated in our previous reports (Krauze et al., 2005b; Mamot et al., 2004; Saito et al., 2004), liposomal gadolinium constructs can be imaged directly in the CNS during CED by MRI, and liposomes containing a marker for imaging can be combined with liposome-encapsulated drugs, enabling real-time guidance of CED (Saito et al., 2005). Although current clinical CED protocols must be improved to translate our recent findings into the clinic, we believe that patients with inoperable glioblastoma are amenable to MRI-guided CED of liposomal drugs aided by the implantation of several catheters within and around tumor tissue. The introduction of our step-design catheter enables especially more precise distribution in the targeted area of the CNS (Krauze et al., 2005b). In our ongoing studies, we have shown that current CED difficulties in clinical trials, such as spillover or reflux in the vicinity of sulci and ventricles, are almost negligible when a step-design catheter is used (unpublished data). Moreover, to prevent relapse, patients undergoing tumor debulking might benefit from postoperative CED for tumor margins. Efficient future brain tumor therapies require three important steps: (1) visualized and controlled direct delivery of therapeutics to CNS, (2) therapeutic combination with high toxicity against malignant cells but low toxicity against healthy brain, and (3) pharmacokinetic profile of therapeutics that enables a long half-life and the introduction of metronomic chemotherapy that targets dormant cancer cells. Considerable progress regarding step 1 has been shown in our previous work. The search for optimal therapeutic combinations is under evaluation and forms part of the current work. Step 3 has been achieved by liposome encapsulation of therapeutics, which has improved the pharmacokinetic profile tremendously in CNS, and it is expected that further research in this expanding field will impact brain tumor treatment modalities. In summary, our approach may represent significant progress toward defining promising chemotherapeutic liposome combinations for future clinical application of CED for the treatment of gliomas.
Acknowledgment
Special thanks are extended to John Forsayeth for proofreading and editing this work.
Footnotes
Grant support for this study was received from the National Cancer Institute Specialized Program of Research Excellence grant (K.S.B. and J.W.P.) and from Accelerate Brain Cancer Cure (K.S.B.), NIH/NCI grant U54 CA90788 (J.W.P.), NIH/NCI contract N01-CO-27031-16 (Hermes Biosciences, Inc.), and CBCRP grant 7KB-0066 (D.C.D.)
Abbreviations used are as follows: BBB, blood-brain barrier; CED, convection-enhanced delivery; DiI-DS, DiIC18(3)-DS; DSPE, disteroyl phosphatidylethanolamine; EC, effective concentration; HBS, HEPES-buffered saline; HBSS, Hanks’ balanced salt solution without Ca2+ and Mg2+; H&E, hematoxylin and eosin; HEPES, N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid); MS, median survival; MTD, maximum tolerated dose; nLs, nanoliposomal; PEG, polyethylene glycol; PLD, pegylated liposomal doxorubicin; TEA8SOS, triethylammonium sucrose octasulfate; topo, topoisomerase; TPT, topotecan.
References
- Allen TM, Martin FJ. Advantages of liposomal delivery systems for anthracyclines. Semin Oncol. 2004;31:5–15. doi: 10.1053/j.seminoncol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Allen TM, Newman MS, Woodle MC, Mayhew E, Uster PS. Pharmacokinetics and anti-tumor activity of vincristine encapsulated in sterically stabilized liposomes. Int J Cancer. 1995;62:199–204. doi: 10.1002/ijc.2910620215. [DOI] [PubMed] [Google Scholar]
- Ando M, Eguchi K, Shinkai T, Tamura T, Ohe Y, Yamamoto N, Kurata T, Kasai T, Ohmatsu H, Kubota K, Sekine I, Hojo N, Matsumoto T, Kodama T, Kakinuma R, Nishiwaki Y, Saijo N. Phase I study of sequentially administered topoisomerase I inhibitor (irinotecan) and topoisomerase II inhibitor (etoposide) for metastatic non-small-cell lung cancer. Br J Cancer. 1997;76:1494–1499. doi: 10.1038/bjc.1997.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Bartlett GR. Colorimetric assay methods for free and phosphorylated glyceric acids. J Biol Chem. 1959;234:469–471. [PubMed] [Google Scholar]
- Berenbaum MC. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JN, Falavigna A, Johnson JP, Hall JS, Birch BD, Yoon JT, Wu EX, Fine RL, Parsa AT. Intracerebral clysis in a rat glioma model. Neurosurgery. 2000;46:683–691. doi: 10.1097/00006123-200003000-00031. [DOI] [PubMed] [Google Scholar]
- Cliby WA, Lewis KA, Lilly KK, Kaufmann SH. S phase and G2 arrests induced by topoisomerase I poisons are dependent on ATR kinase function. J Biol Chem. 2002;277:1599–1606. doi: 10.1074/jbc.M106287200. [DOI] [PubMed] [Google Scholar]
- Degen JW, Walbridge S, Vortmeyer AO, Oldfield EH, Lonser RR. Safety and efficacy of convection-enhanced delivery of gemcitabine or carboplatin in a malignant glioma model in rats. J Neurosurg. 2003;99:893–898. doi: 10.3171/jns.2003.99.5.0893. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–743. [PubMed] [Google Scholar]
- Drummond DC, Marx C, Guo Z, Scott G, Noble C, Wang D, Pallavicini M, Kirpotin DB, Benz CC. Enhanced pharmacodynamic and antitumor properties of a histone deacetylase inhibitor encapsulated in liposomes or ErbB2-targeted immunoliposomes. Clin Cancer Res. 2005;11:3392–3401. doi: 10.1158/1078-0432.CCR-04-2445. [DOI] [PubMed] [Google Scholar]
- Eder JP, Chan V, Wong J, Wong YW, Ara G, Northey D, Rizvi N, Teicher BA. Sequence effect of irinotecan (CPT-11) and topoisomerase II inhibitors in vivo. Cancer Chemother Pharmacol. 1998;42:327–335. doi: 10.1007/s002800050825. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: Review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- Groothuis DR. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-Oncology. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross MW, Altscher R, Brandtner M, Haeusser-Mischlich H, Chiricuta IC, Siegmann AD, Engenhart-Cabillic R. Open-label simultaneous radio-chemotherapy of glioblastoma multiforme with topotecan in adults. Clin Neurol Neurosurg. 2005;107:207–213. doi: 10.1016/j.clineuro.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hammond LA, Eckardt JR, Ganapathi R, Burris HA, Rodriguez GA, Eckhardt SG, Rothenberg ML, Weiss GR, Kuhn JG, Hodges S, Von Hoff DD, Rowinsky EK. A phase I and translational study of sequential administration of the topoisomerase I and II inhibitors topotecan and etoposide. Clin Cancer Res. 1998;4:1459–1467. [PubMed] [Google Scholar]
- Hau P, Fabel K, Baumgart U, Rümmele P, Grauer O, Bock A, Dietmaier C, Dietmaier W, Dietrich J, Dudel C, Hübner F, Jauch T, Drechsel E, Kleiter I, Wismeth C, Zellner A, Brawanski A, Steinbrecher A, Marienhagen J, Bogdahn U. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer. 2004;100:1199–1207. doi: 10.1002/cncr.20073. [DOI] [PubMed] [Google Scholar]
- Herben VM, ten Bokkel Huinink WW, Dubbelman AC, Mandjes IA, Groot Y, van Gortelvan Zomeren DM, Beijnen JH. Phase I and pharmacological study of sequential intravenous topotecan and oral etoposide. Br J Cancer. 1997;76:1500–1508. doi: 10.1038/bjc.1997.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MG, Parsa AT, Fine RL, Hall JS, Chakrabarti I, Bruce JN. Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery. 2000;47:1391–1398. [PubMed] [Google Scholar]
- Kaufmann SH, Peereboom D, Buckwalter CA, Svingen PA, Grochow LB, Donehower RC, Rowinsky EK. Cytotoxic effects of topotecan combined with various anticancer agents in human cancer cell lines. J Natl Cancer Inst. 1996;88:734–741. doi: 10.1093/jnci/88.11.734. [DOI] [PubMed] [Google Scholar]
- Kim R, Hirabayashi N, Nishiyama M, Jinushi K, Toge T, Okada K. Experimental studies on biochemical modulation targeting topoisomeraseI and IIin human tumor xenograftsin nudemice. Int J Cancer. 1992;50:760–766. doi: 10.1002/ijc.2910500516. [DOI] [PubMed] [Google Scholar]
- Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, Berger MS, Bankiewicz K. Reflux-free cannula for convection-enhanced high-speed delivery for therapeutic agents. J Neurosurg. 2005a;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauze MT, Mcknight TR, Yamashita Y, Bringas J, Noble CO, Saito R, Geletneky K, Forsayeth J, Berger MS, Jackson P, Park JW, Bankiewicz KS. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005b;16:20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- MacKay JA, Deen DF, Szoka FC., Jr Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035:139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Mamot C, Nguyen JB, Pourdehnad M, Hadaczek P, Saito R, Bringas JR, Drummond DC, Hong K, Kirpotin DB, McKnight T, Berger MS, Park JW, Bankiewicz KS. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68:1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- Noble CO, Krauze MT, Drummond DC, Yamashita Y, Saito R, Berger MS, Kirpotin DB, Bankiewicz KS, Park JW. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: Pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- Penson RT, Seiden MV, Goodman A, Fuller AF, Jr, Berkowitz RS, Matulonis UA, Krasner C, Lee H, Atkinson T, Campos SM for the Gynecologic Oncology Research Program at Dana Farber/Partners CancerCare. Phase I trial of escalating doses of topotecan in combination with a fixed dose of pegylated liposomal doxorubicin in women with mullerian malignancies. Gynecol Oncol. 2004;93:702–707. doi: 10.1016/j.ygyno.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Pipas JM, Meyer LP, Rhodes CH, Cromwell LD, McDonnell CE, Kingman LS, Rigas JR, Fadul CE. A phase II trial of paclitaxel and topotecan with filgrastim in patients with recurrent or refractory glioblastoma multiforme or anaplastic astrocytoma. J Neuro-oncol. 2005;71:301–305. doi: 10.1007/s11060-004-2026-2. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Kaufmann SH. Topotecan in combination chemotherapy. Semin Oncol. 1997;24(suppl):S20-11–S20-S26. [PubMed] [Google Scholar]
- Saito R, Bringas JR, McKnight TR, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64:2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Hong K, Berger MS, Park JW, Bankiewicz KS. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196:381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Saito R, Krauze MT, Noble CO, Drummond DC, Kirpotin DB, Berger MS, Park JW, Bankiewicz KS. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro-Oncology. 2006a;8:205–214. doi: 10.1215/15228517-2006-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R, Krauze MT, Noble CO, Tamas M, Drummond DC, Kirpotin DB, Berger MS, Park JW, Bankiewicz KS. Tissue affinity of the infusate affects distribution volume during convection-enhanced delivery into rodent brains: Implications for local drug delivery. J Neurosci Methods. 2006b;154:225–232. doi: 10.1016/j.jneumeth.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Sinha BK. Topoisomerase inhibitors. A review of their therapeutic potential in cancer. Drugs. 1995;49:11–19. doi: 10.2165/00003495-199549010-00002. [DOI] [PubMed] [Google Scholar]
- Taron M, Plasencia C, Abad A, Martin C, Guillot M. Cytotoxic effects of topotecan combined with various active G2/M-phase anticancer drugs in human tumor-derived cell lines. Invest New Drugs. 2000;18:139–147. doi: 10.1023/a:1006325929424. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Saito R, Krauze MT, Kawaguchi T, Noble C, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS. Convection-enhanced delivery of liposomal doxorubicin in intracranial brain tumor xenografts. Targeted Oncol. 2006;1:79–85. [Google Scholar]
- Zhou R, Mazurchuk R, Straubinger RM. Antivasculature effects of doxorubicin-containing liposomes in an intracranial rat brain tumor model. Cancer Res. 2002;62:2561–2566. [PubMed] [Google Scholar]