Abstract
During phagocytosis, macrophages rapidly internalize a substantial fraction of plasma membrane without a net loss of surface area, suggesting that membranes are targeted to the cell surface from intracellular sites. Nevertheless, a requirement for mobilization of specific membrane compartments has not been demonstrated. We used bone marrow-derived macrophages (BMM) from wild type and vamp3 null mice to evaluate directly the requirement for this v-SNARE in phagocytosis of zymosan, IgG-beads, complement-opsonized particles, or latex microspheres. Regardless of the phagocytic receptor engaged or particle load, BMM lacking vamp3 exhibited no phagocytic defects when assayed after 1 h at 37°C, and phagosome maturation was unimpaired as judged by acquisition of lamp-1. In contrast, at early time points (5–15 min), internalization of zymosan (but not other particles tested) was significantly slower in vamp3 null BMM. These data indicate that vamp3 modulates efficient uptake of zymosan, but is not absolutely required for phagocytosis in primary macrophages.
Keywords: phagosome, exocytosis, cellubrevin, v-SNARE, IgG-beads
INTRODUCTON
The ability to ingest microbes and other large particles is unique to phagocytic cells (macrophages, monocytes, and neutrophils) and is an essential component of the immune response. Like receptor-mediated endocytosis, phagocytosis requires receptor-ligand interactions; however, in contrast to endocytosis, particle internalization is driven by localized actin polymerization rather than by clathrin assembly [1]. One characteristic of phagocytes is their ability to ingest large numbers of particles or microbes quickly, suggesting that these cells have the capacity to rapidly replenish or expand their plasma membranes. Recent data indicate that during phagocytosis, macrophage surface area increases [2, 3], but the source(s) of membrane for this expansion remains undefined. A role for vesicle-associated, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (v-SNAREs) in plasma membrane expansion during phagocytosis is suggested by the observation that vesicle-associated membrane protein 3 (vamp3, also called cellubrevin) is enriched on early phagosomes in the murine macrophage cell line J774 and in nonprofessional phagocytes [Chinese hamster ovary cells (CHO)] transfected with the Fcγ receptor FcγRIIA [4]. Moreover, microinjection of tetanus or botulinum toxins (which cleave and degrade vamp2 and vamp3) reduced phagocytosis of a variety of particles by ~66% in J774 cells [2]. Nonetheless, a requirement for specific v-SNAREs during phagocytosis has not been demonstrated.
In the current study, the role of the v-SNARE vamp3 in phagocytosis was evaluated using macrophages from vamp3 null mice. We now show that vamp3 is essential for optimal phagocytosis of zymosan following engagement of mannose and β-glucan receptors, but does not affect macrophage-phagocytic capacity or phagosome maturation.
MATERIALS AND METHODS
Trypticase soy agar was obtained from Difco Laboratories (Detroit, MI). Pyrogen-free tissue-culture reagents, Dulbecco’s phosphate-buffered saline, Hepes-RPMI, alpha modified Eagle’s medium (MEMα), L-glutamine, and penicillin-streptomycin, were from BioWhittaker (Walkersville, MD). Fetal bovine serum (FBS) was from HyClone (Logan, UT) or Gibco (Grand Island, NY). Round glass coverslips (12 mm) were from Fisher (Pittsburgh, PA). Fluorescent zymosan particles and zymosan opsonizing reagent [immunoglobulin G (IgG)] were from Molecular Probes (Eugene, OR). Mouse hybridoma supernatant to lamp-1 was from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City). Affinity-purified fluorescein isothiocyanate (FITC)- and tetramethyl rhodamine isothiocyanate-conjugated donkey anti-rabbit IgG F(ab′)2 and FITC-conjugated goat anti-mouse or goat anti-rat IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA). Latex beads (1 μm diameter) were the generous gift of Dr. Larry Schlesinger (Department of Medicine, University of Iowa). Polyvinylidene difluoride membranes were from Millipore (Bedford, MA). Horseradish peroxidase-conjugated antibodies were from Biorad (Hercules, CA). Coomassie Plus and bicinchoninic acid protein assay kits and Super Signal enhanced chemiluminescence (ECL) reagents were from Pierce (Rockford, IL). Monoclonal antibodies (mAb) to talin and additional reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
Macrophage isolation and culture
Generation of vamp3 null mice has been described [5]. Bone marrow-derived macrophages (BMM) were cultured from wild-type (wt) and vamp3 null mice. Marrow was flushed from excised femurs and cultured at 37°C in complete medium [Hepes-RPMI supplemented with 15% heat-inactivated (HI)-FBS, 1% L-glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 20% L cell-conditioned medium, a source of colony-stimulating factor type 1 (CSF-1)]. After 48 h at 37°C, culture supernatants containing macrophage progenitor cells were transferred to petri dishes (bacteriologic plastic). Cells were fed fresh L929 cell-conditioned medium each 48 h, and mature BMM were used after a total of 7–19 days in culture. For each experiment, BMM were scraped off petri dishes and replated on coverslips or in tissue culture dishes as indicated. BMM were switched, 12–24 h after plating, to MEMα containing 10% HI-FBS and 1% L-glutamine (without antibiotics or CSF-1) and were incubated overnight at 37°C prior to use.
Western blotting
Immunodetection of vamp2 and vamp3 in macrophage lysates was performed as we described [5] using biotinylated anti-vamp3 IgG or rabbit anti-vamp2 antiserum. Bands were visualized using ECL reagents.
Synchronized phagocytosis
Unopsonized zymosan particles and zymosan particles opsonized with complement (COZ) were prepared as described [6]. IgG-opsonized zymosan particles were prepared using Molecular Probes opsonizing reagent according to the manufacturer’s specifications. IgG-coated magnetic beads were obtained from Dynal (Lake Success, NY). Washed particles were dispersed in tissue culture medium to achieve a ratio of 3 zymosan particles, 5 IgG-beads, or 10 latex beads per macrophage. For all experiments, phagocytosis was synchronized using centrifugation [6].
Immunofluorescence microscopy
Macrophages were plated on acid-washed glass coverslips (35,000 BMM each) in complete medium and then starved of CSF-1 and antibiotics as indicated above. Attachment indices (total particles per 100 macrophages) and phagocytic indices (phagosomes per 100 macrophages) were scored as described [6, 7]. For each experiment, at least 100 infected macrophages were counted on triplicate coverslips. Kinetics of phagocytosis were determined by staining fixed and permeabilized cells with anti-talin mAb and secondary antibodies conjugated to FITC [7]. Phagosome fusion with late endosomes/lysosomes was detected by staining fixed and permeabilized cells with rat mAb to lamp-1 and secondary antibodies conjugated to FITC [7]. Particles were detected by phase contrast optics, and recruitment of marker proteins to phagosomes was assessed using a Zeiss Axioplan2 fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY). For each experiment, phagosomes in at least 100 infected cells on each of three coverslips were scored for the presence of lamp-1 or talin. To assess phagocytic capacity, particle load per cell was increased to ~ 65 zymosan or opsonized zymosan particles or ~125 latex beads per BMM. Where indicated, exposure of fluorescent zymosan particles to the extracellular milieu was assessed using trypan blue quenching [8]. Composite images were generated using Adobe Photoshop 3.0 (Adobe Systems Inc., Mountain View, CA).
RESULTS
Vamp3 is not essential for phagocytosis
BMM were cultured from wt or vamp3 null mice [5]. Western blotting of clarified lysates demonstrated that vamp3 was abundant in wt BMM but was undetectable in BMM from vamp3 null mice (Fig. 1A and ref [5]). Ablation of vamp3 did not alter expression of vamp2, syntaxin 4, or other proteins involved in endocytic membrane trafficking and fusion (Fig. 1A and ref. [5]). To determine whether vamp3 was essential for phagocytosis, we assessed the ability of vamp3 null and wt BMM to ingest a variety of particles that engage distinct phagocytic receptors: IgG-beads, which bind FcγR; zymosan particles, which bind mannose and β-glucan receptors; complement-opsonized particles, which bind CD11b/CD18; and latex beads, which bind an unidentified receptor [1, 6]. In all cases, phagocytosis was synchronized using centrifugation, and internalization of bound particles was assayed after 60 min at 37°C. As shown in Figure 1B and C, we found that wt and vamp3 null BMM bound and ingested all particles tested, regardless of the phagocytic receptor engaged. These data suggest that vamp3 is not essential for phagocytosis in BMM.
Fig. 1.
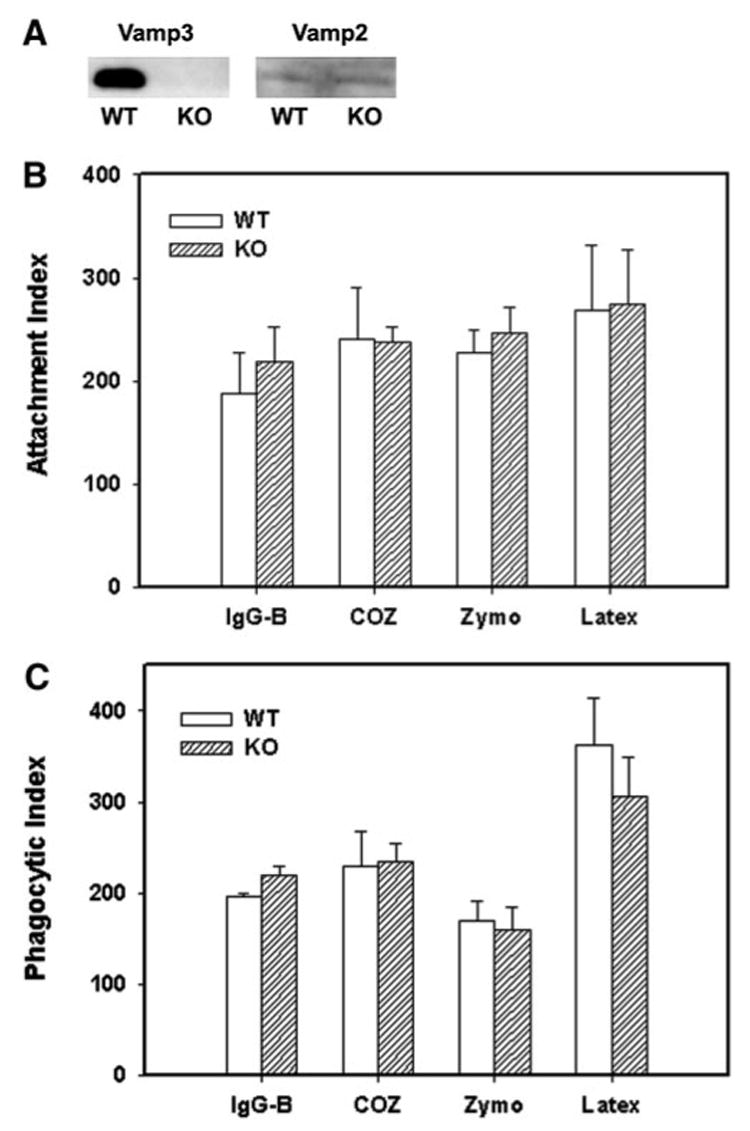
Vamp3 null BMM phagocytose a variety of particles. (A) vamp3 and vamp2 content of wt and mutant macrophages (KO) as judged by Western blotting of clarified lysates. (B, C) IgG-beads (IgG-B), COZ, unopsonized zymosan (Zymo), or latex beads were added to adherent wt (WT) or vamp3 null (KO) BMM, and after 1 h at 37°C, total cell-associated particles (B) and total phagosomes (C) per 100 cells were scored as described in Materials and Methods. Data shown are the average ± SD of six independent experiments conducted in triplicate.
Zymosan phagocytosis is retarded in the absence of vamp3
Localized actin polymerization is required for phagocytosis, and cytoskeletal proteins such as talin are also recruited to, and enriched on, forming phagosomes [6, 7, 9]. Once particle internalization is complete, cytoskeletal proteins are shed from nascent phagosomes, thereby permitting interaction and fusion of these compartments with other organelles [6, 7]. To determine whether phagocytic rate or efficiency was regulated by vamp3, we followed the kinetics of talin association with forming phagosomes in wt and vamp3 null BMM. Consistent with our previous findings using peritoneal macrophages [6, 7], talin-positive-forming phagosomes containing zymosan, IgG-beads, or COZ were abundant within 1 min of incubation at 37°C, and talin was shed from the phagosome membrane after particle internalization was complete (Fig. 2). According to this assay, IgG-beads and COZ were ingested with equal efficiency by BMM, regardless of vamp3 content (Fig. 2B and C).
Fig. 2.
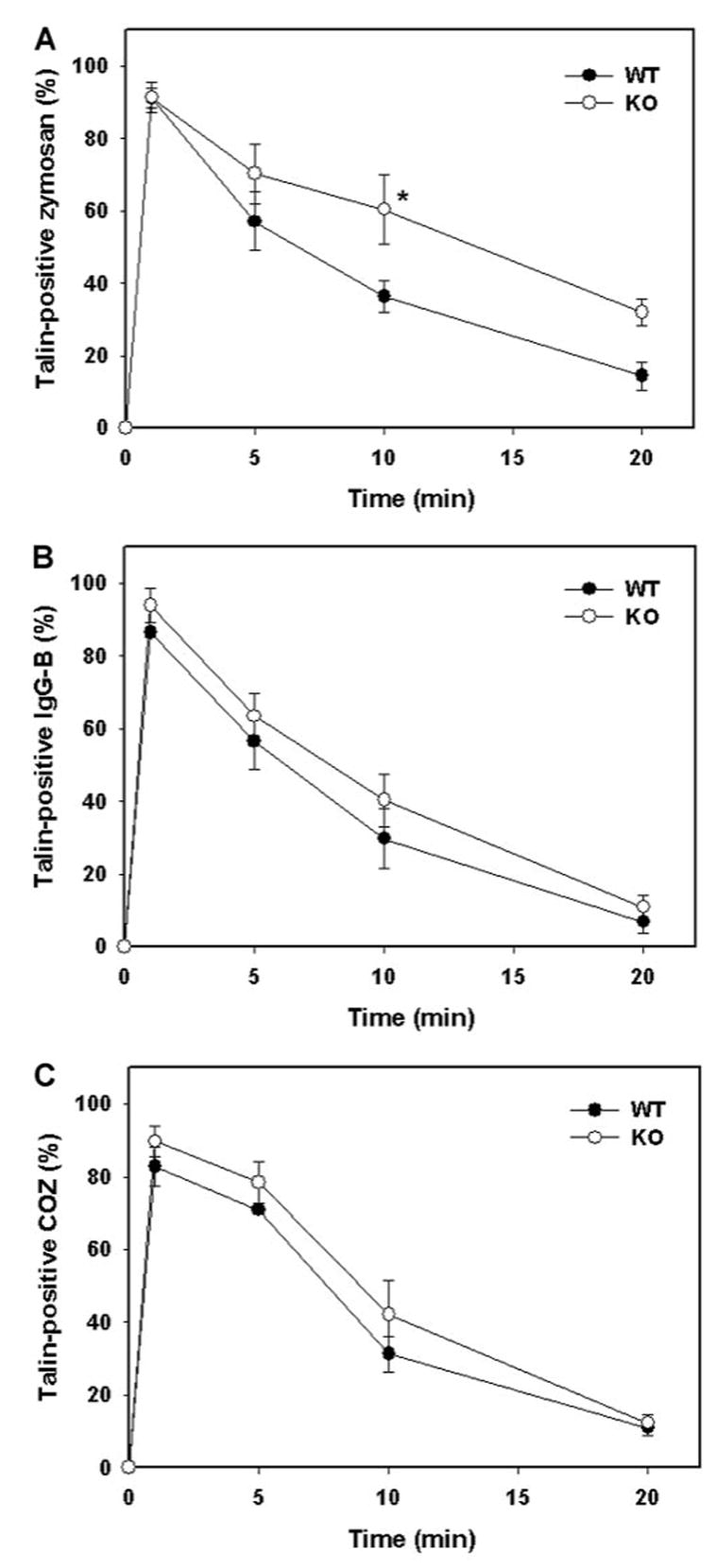
Zymosan uptake is retarded in cells lacking vamp3. Phagocytosis of zymosan (A), IgG-beads (B), or COZ (C) by adherent wt or vamp3 null (KO) BMM was synchronized using centrifugation [6]. After 1–20 min at 37°C, samples were fixed and permeabilized, then stained with mAb to talin, and secondary antibodies conjugated to FITC. Graphs show the time course of talin association with forming phagosomes and are the average ± SD of four independent experiments conducted in triplicate. (●) wt BMM; (○) vamp3 null BMM. *, Statistical significance (P<0.05; Student’s t-test) relative to wt.
In contrast, phagocytosis of zymosan was significantly slower in macrophages lacking vamp3. Although forming phagocytic cups were apparent in wt and vamp3 null macrophages after 1 min at 37°C (Fig. 3A), differences in zymosan uptake were apparent within 5 min and were most pronounced after 10 min (Fig. 2A). Indeed, as judged by talin immunofluorescence at 10 min, 63.6 ± 4.5% versus 39.6 ± 8.7% of cell-associated zymosan particles was intracellular in wt and vamp3 mutant cells, respectively. To assess whether vamp3 was affecting zymosan internalization directly (and not retarding shedding of cytoskeletal proteins from nascent phagosomes), phagocytosis was also followed using an independent assay in which fluorescence of extracellular or surface-exposed particles is quenched by trypan blue [8]. According to this assay, the half-time for zymosan phagocytosis was increased by ~52% in the absence of vamp3 (9.5 min and 14.5 min in wt and vamp3 null macrophages, respectively).
Fig. 3.
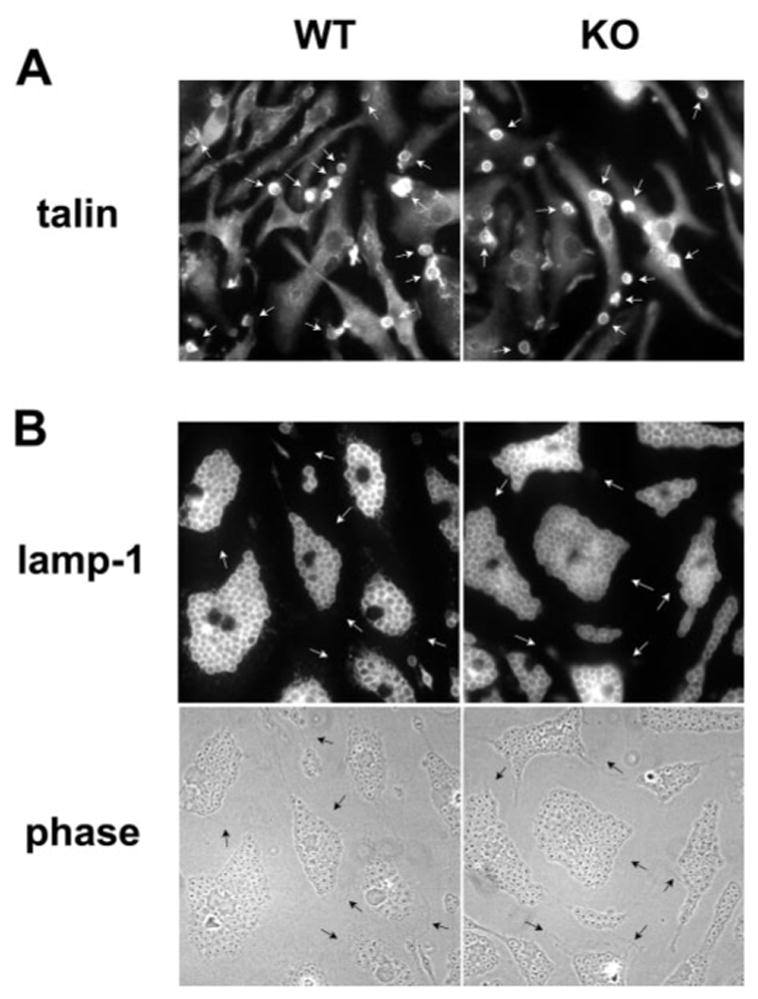
Phagocytic capacity of vamp3 null BMM is not impaired. (A) Forming zymosan phagosomes (30 s time point) are enriched for talin in wt and vamp3 null (KO) BMM (arrows). (B) One hour after addition of ~65 COZ per cell, BMM were fixed and stained with antibodies to lamp-1. Note that vamp3 null and wt BMM ingested numerous COZ as judged by phase contrast optics (lower panels) and lamp-1 fluorescence (upper panels). Arrows indicate cell margins.
Phagocytic capacity of vamp3 null macrophages
In the experiments noted above, particle load was relatively low, and it is possible that a requirement for vamp3 might be more apparent when phagocytic load is very high. To test this possibility, wt and vamp3 BMM were incubated for 1 h at 37°C with a ratio of ~65 zymosan or opsonized-zymosan particles (4.5 μm diameter) or 125 latex beads (1 μm diameter) per cell, and phagocytosis was monitored using antibodies to lamp-1 and phase-contrast optics [7]. As shown in Figure 3B, vamp3 null and wt BMM ingested COZ until the cytoplasm was almost full of particles (~50 phagosomes/cell). Comparable data were also obtained using unopsonized zymosan, IgG zymosan, or latex beads (Table 1). In spite of the high particle load, >98% of all cell-associated particles were ingested. Moreover, the vast majority of phagosomes matured normally, as judged by staining with antibodies to the late endosome/lysosome marker lamp-1 (<1% vs. 2.3 ± 1.6% lamp-1-negative phagosome in wt and vamp3 null BMM, respectively; Fig. 3B). Taken together, the data indicate that vamp3 is required for optimal phagocytosis of zymosan, but does not regulate macrophage-phagocytic capacity or phagosome maturation.
TABLE 1.
Macrophage Phagocytic Capacity
| Particle | Wild-type | vamp3 null |
|---|---|---|
| Zymo. | 37 ±7 | 48 ± 9 |
| IgG-Zymo. | 47 ±8 | 56 ± 10 |
| COZ | 64 ± 7 | 52 ± 8 |
| Latex | > 100 | > 100 |
wt and vamp3 null BMM were incubated with ~65 zymosan or opsonized zymosan particles or ~125 latex beads per cell for 1 h at 37°C. Data shown indicate the number of ingested particles per BMM (average ± SD) from three independent experiments.
DISCUSSION
One of the unresolved questions in the field of phagocytosis is how cell surface area is replenished in spite of substantial membrane internalization. The speed and efficiency of particle internalization preclude a direct role for membrane synthesis and suggest a requirement for mobilization of membrane from intracellular sites. However, neither the nature of the mobilized membrane nor its regulation has been defined. In this regard, recent studies of CHO transfected with FcγRIIA demonstrated that vamp3 is recruited to forming phagosomes from an intra-cellular compartment [4]. Herein, we explored the role of vamp3 directly in macrophage phagocytosis and present four lines of evidence, which indicate that vamp3 modulates only a subset of phagocytic processes. First, vamp3 null macrophages bound to and ingested a variety of particles (Fig. 1) and microbes (data not shown). Second, the rate of zymosan phagocytosis, but not uptake of IgG-beads or COZ, was significantly impaired in the absence of vamp3 (Fig. 2). Third, macrophage capacity to ingest large numbers of particles was not altered (Table 1). Fourth, the absence of vamp3 did not inhibit phago-some maturation as judged by acquisition of the late endosomelysosome marker lamp-1 (Fig. 3B).
The lack of a gross phagocytic defect in vamp3 null macrophages suggests that other v-SNAREs may regulate membrane addition at the cell surface. Although it is less abundant than vamp3, macrophages also contain vamp2 [2], and our observation that vamp2 levels were unaltered in vamp3 null BMM suggests that vamp2 may regulate exocytic events that accompany phagocytosis under basal conditions and in the absence of vamp3. Consistent with this hypothesis, neutrophils contain vamp2 but lack vamp3 [10]. To date, recruitment of vamp2 or other SNAREs to macrophage phagosomes has not been demonstrated, and unfortunately, our antibodies to vamp2 and vamp3 were not suitable for immunofluorescence microscopy (unpublished results). Nevertheless, it has been documented that vamp2, but not vamp3, is essential for fusion of GLUT4-containing vesicles with the plasma membrane of insulin-stimulated myoblasts [11].
Dissection of the role of individual v-SNAREs in phagocytosis is complicated by the fact that the mobilized membrane compartments have not been defined precisely. Recycling en-dosomes are an attractive candidate for several reasons. First, recycling endosomes mediate membrane trafficking between early endosomes and the cell surface, and membrane vesicles accumulate in the periphagosomal area during particle ingestion [6]. Second, in contrast to other cell types, recycling endosomes are found throughout the macrophage cytoplasm [12]. Third, vamp2, vamp3, vamp8, and rab11 are all associated with recycling endosomes [12–14]. Finally, independent studies have shown that tetanus toxin-mediated cleavage of vamp2 and vamp3 or expression of mutant forms of rab11 in macrophage cell lines reduces phagocytosis by 50 –66% [2, 12]. However, the fact that neither of these approaches [nor elimination of vamp3 (this study)] ablates particle ingestion suggests that vamp2, −3, and −8 may play redundant roles in phagocytosis. Alternatively, other organelles may contribute to plasma membrane expansion during phagocytosis. Indeed, an elegant study by Garin et al. [15] suggests a central role for the endoplasmic reticulum (but not the Golgi) in formation of phagosomes containing latex beads. Precise characterization of the composition of forming phagosomes and studies using macrophages with single or double mutations in v-SNAREs associated with distinct membrane compartments will be required to resolve this issue.
Our finding that vamp3 was dispensable for phagocytosis of COZ, IgG-, and latex beads, but not unopsonized zymosan, suggests that vamp3-containing membranes may be preferentially mobilized by signals generated following engagement of mannose and/or β-glucan receptors. Cross-linking FcγR activates syk and multiple src family protein tyrosine kinases, phosphoinositide 3-kinase, protein kinase C (PKC), and the small molecular weight GTPases rac and cdc42, which together orchestrate localized rearrangements of the actin cytoskeleton and uptake of IgG-coated particles [3, 6, 16–24]. In contrast, the signaling pathways downstream of other phagocytic receptors are less well defined. CD11b/ CD18-mediated phagocytosis requires activation of PKC and the small GTPase rho (but not rac or cdc42) yet is relatively insensitive to inhibitors of tyrosine kinases [1, 6, 24]. Conversely, phagocytosis of zymosan and other yeast particles requires PKC and a distinct subset of src family kinases but is independent of syk [7, 18, 20]. Moreover, we have shown that forming zymosan phagosomes accumulate a more restricted subset of cytoskeletal proteins than do phagosomes containing IgG-beads or COZ [6]. Thus, it is tempting to speculate that vamp3 is preferentially mobilized during phagocytosis of zymosan. Whether recruitment of vamp3 to forming phagosomes requires PKC is unknown; however, PKC-dependent signaling has long been known to regulate exocytic events in other systems including degranulation of human neutrophils [25].
CONCLUSIONS
In conclusion, we have shown that the v-SNARE vamp3 is dispensable for phagosome maturation yet is required for the rapid and efficient uptake of zymosan particles by primary BMM. Our data underscore the fact that multiple pathways for phagocytosis exist in macrophages and further suggest that vamp3-negative organelles may play a significant role in phagosome expansion downstream of FcγR and complement receptors. Precise definition of the membrane compartments mobilized during phagocytosis and generation of macrophages with mutations in other v-SNAREs will be required to delineate further the molecular mechanisms of phagocytosis.
Acknowledgments
This work was supported in part by funds to from the Department of Veterans Affairs and the Public Health Service (National Institutes of Health R01 AI43617) to L-A. H. A.
References
- 1.Greenberg S, Silverstein SC. Phagocytosis. In: Paul WE, editor. Fundamental Immunology. New York: Raven; 1993. pp. 941–964. [Google Scholar]
- 2.Hackam DJ, Rotstein OD, Sjolin C, Schreiber AD, Trimble WS, Grinstein S. v-SNARE-dependent secretion is required for phagocytosis. Proc Natl Acad Sci USA. 1998;95:11691–11696. doi: 10.1073/pnas.95.20.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240 –1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 4.Banjo L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of vamp3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–705. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Mora S, Ryder JW, Coker KJ, Hansen P, Allen LA, Pessin JE. VAMP3 null mice display normal constitutive, insulin- and exercise-regulated vesicle trafficking. Mol Cell Biol. 2001;21:1573–1580. doi: 10.1128/MCB.21.5.1573-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen LAH, Aderem A. Molecular definition of distinct cy-toskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen LAH, Aderem A. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med. 1995;182:829 –840. doi: 10.1084/jem.182.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan CP, Park CS, Lau BH. A rapid and simple microfluorometric phagocytosis assay. J Immmunol Methods. 1993;162:1–7. doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg S, Burridge K, Silverstein SC. Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1990;172:1853–1856. doi: 10.1084/jem.172.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brumell JH, Sengelov H, Borregaard N, Cieutat AM, Bainton DF, Grinstein S, Klip A. J Immunol. 1995;155:5750–5759. [PubMed] [Google Scholar]
- 11.Randhawa VK, Bilan PJ, Khayat ZA, Daneman N, Liu Z, Ramlal T, Volchuk A, Peng XR, Coppola T, Regazzi R, Trimble WS, Klip A. VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol Biol Cell. 2000;11:2403–2417. doi: 10.1091/mbc.11.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci USA. 2000;97:680 –685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verges M, Havel RJ, Mostov KE. A tubular endosomal fraction from rat liver: biochemical evidence of receptor sorting by default. Proc Natl Acad Sci USA. 1999;96:10146 –10151. doi: 10.1073/pnas.96.18.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wit H, Lichtenstein Y, Geuze HY, Kelly RB, van der Sluijs P, Klumperman J. Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells. Mol Biol Cell. 1999;10:4163–4176. doi: 10.1091/mbc.10.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garin J, Diez RD, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox D, Chang P, Kurosaki T, Greenberg S. Syk tyrosine kinase is required for immunoreceptor tyrosine activation motif-dependent actin assembly. J Biol Chem. 1996;271:16597–16602. doi: 10.1074/jbc.271.28.16597. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg S, Chang P, Wang DC, Xavier R, Seed B. Clustered syk tyrosine kinase domains trigger phagocytosis. Proc Natl Acad Sci USA. 1996;93:1103–1107. doi: 10.1073/pnas.93.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng FY, DeFranco AL, Lowell CA. Fcγ receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669 –681. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majeed M, Caveggion E, Lowell CA, Berton G. Role of Src kinases and Syk in Fcγ receptor-mediated phagocytosis and phagosomelysosome fusion. J Leukoc Biol. 2001;70:801–811. [PubMed] [Google Scholar]
- 21.Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation of the gamma subunit of Fc receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J Biol Chem. 1994;269:3897–3902. [PubMed] [Google Scholar]
- 22.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249 –1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheleznyak A, Brown EJ. Immunoglobulin-mediated phagocytosis by human monocytes requires protein kinase C activation. Evidence for protein kinase C translocation to phagosomes. J Biol Chem. 1992;267:12042–12048. [PubMed] [Google Scholar]
- 24.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 25.White JR, Huang CK, Hill JM, Jr, Maccache PH, Becker EL, Sha’afi RI. Effect of phorbol 12-myristate 13-acetate and its analogue 1 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984;259:8605–8611. [PubMed] [Google Scholar]


