Abstract
Objective:
To compare the occurrence of normal visual field (VF) tests following 2 versus 3 consecutive, abnormal reliable tests in the Ocular Hypertension Treatment Study (OHTS).
Methods:
The OHTS is a prospective, multicenter, follow-up study as part of a longitudinal randomized clinical trial. Sixty-four participants (68 eyes) developed a VF primary open-angle glaucoma (POAG) endpoint from 1,636 participants. We compared the proportion of normal VF tests after a VF POAG endpoint among eyes whose VF abnormality was confirmed by 2 (n=9 eyes) vs. 3 (n=59 eyes) consecutive, abnormal reliable VF tests.
Results:
The proportion of VF tests that were normal subsequent to a VF POAG endpoint in eyes whose abnormality was confirmed by 2 consecutive, abnormal reliable tests was significantly higher (73 [66%] of 110) compared to eyes whose abnormality was confirmed by 3 consecutive, abnormal reliable tests (46 [12%] of 381) (P = 0.013).
Conclusion:
A VF POAG endpoint confirmed by 3 consecutive, abnormal reliable VF tests appears to have greater specificity and stability than either 1 or 2 consecutive, abnormal reliable VF tests. However, some eyes whose VF POAG endpoint was confirmed by 3 consecutive, abnormal reliable tests nonetheless had one or more normal tests on follow-up.
Introduction
The Ocular Hypertension Treatment Study (OHTS) is a prospective, longitudinal, randomized clinical trial to evaluate the safety and efficacy of topical ocular hypotensive medication in delaying or preventing the onset of glaucomatous visual field loss and/or optic nerve deterioration in patients at moderate risk for developing primary open-angle glaucoma (POAG). Results of the OHTS trial have been previously published.1
According to OHTS criteria, a visual field is considered abnormal if the Glaucoma Hemifield Test (GHT) is outside of normal limits and/or the Corrected Pattern Standard Deviation (CPSD) is P < 5%. Originally, the OHTS criteria for confirmation of a visual field abnormality (endpoint) required two consecutive, abnormal, and reliable visual field tests, with the abnormality in the same location and on the same index. During OHTS follow up, a high percentage (85.9%) of the visual field tests immediately following the first abnormal visual field were normal.2 The low degree of reproducibility, as well as maintaining a high specificity for determining POAG visual field endpoints, suggested the need for additional abnormal visual field tests and a longer waiting period between tests before declaring the patient at a visual field endpoint. Thus, at the recommendation of the OHTS Data and Safety Monitoring Committee, the OHTS Steering Committee, and the OHTS Full Investigator Group, the protocol was changed for confirmation of visual field abnormality (endpoint) on June 1, 1997 to require three consecutive, abnormal and reliable visual field tests with the defect in the same location and on the same index.2 In this paper we report how often subsequent visual field tests were normal in OHTS participants who reached a visual field POAG endpoint based on 2 versus 3 consecutive, abnormal, and reliable visual field tests.
Methods
A total of 1,636 participants who met entry criteria and had no evidence of glaucomatous damage (ages 40 to 80 years) and with intraocular pressures (IOP) between 24 mm Hg and 32 mm Hg in one eye and between 21 mm Hg and 32 mm Hg in the other eye, were randomized to either observation or treatment with commercially available topical ocular hypotensive medication.1 Institutional Review Board approval and Informed Consents were obtained (in accordance with CFR 45 regulations) prior to participant recruitment at each clinical center.
Participants underwent at least two Humphrey Field Analyzer (HFA) Program 30-2 Full Threshold visual field examinations in both eyes to determine visual field eligibility. A third examination was performed if one of the two prior tests was abnormal, questionable, or unreliable. For final visual field eligibility, the field tests had to be normal and reliable in both eyes on two examinations, as determined by the Visual Field Reading Center (VFRC). The optic nerve heads also had to be normal in both eyes on clinical examination and review of stereoscopic optic disc photographs, as determined by the Optic Disc Reading Center (ODRC). Follow-up visual field examinations were obtained at six-month intervals.1 Neither the participants nor the clinicians were masked to the randomization assignments during follow up.
According to OHTS criteria, a reliable visual field was defined as abnormal if the Glaucoma Hemifield Test (GHT) was outside of normal limits and/or the Corrected Pattern Standard Deviation (CPSD) was P < 5%. Initially, the OHTS protocol for confirmation of a visual field abnormality required two consecutive, abnormal, and reliable visual field tests, with the abnormality in the same index and in the same location (involving similar points as the previous visual field). The second visual field test was performed within 1 day to 8 weeks of the first abnormal visual field test. A visual field abnormality was not considered confirmed if it was judged to be artifactual by the VFRC readers (i.e. trial lens rim artifacts that disappeared on retest or superior depression that disappeared with taping of the eyelid).
The protocol for confirming visual field abnormality was changed on June 1st, 1997, to require three consecutive, abnormal and reliable visual field tests with the defect in the same location and on the same index.2,3 Thus, a participant with an abnormal visual field test was tested at the next regularly scheduled follow-up visit in 6 months. If the VFRC considered the second visual field test abnormal, it requested a third visual field to be completed in 1 day to 8 weeks. If the third visual field test was considered by the VFRC Director and Co-Director (JK and CJ) to have confirmed the visual field abnormality, the VFRC then prepared a narrative description of the abnormality and sent all visual field tests to the OHTS Coordinating Center for review by the OHTS Endpoint Committee. The Endpoint Committee members made an independent determination as to whether the visual field abnormality was attributable to POAG after carefully reviewing all the optic disc photographs, visual field tests, and medical and ocular history from baseline of both eyes of the participant.1 Disagreement between committee members was resolved by consensus. Committee members were masked as to the POAG classification of the affected and fellow eye, randomization status, and previous intraocular surgery.
In this report, we included POAG visual field data through November 8, 2001. Sixty-eight eyes reached a visual field POAG endpoint; 9 eyes based on the confirmation criteria of two consecutive, abnormal, and reliable visual field tests, and 59 eyes based on the confirmation criteria of three consecutive, abnormal, and reliable visual field tests. Analysis of these data was complicated by two factors. First, four participants reached POAG visual field endpoints in both eyes. One of the eight eyes reached endpoint based on two consecutive, abnormal, and reliable visual field tests and the other seven eyes reached endpoint based on three consecutive, abnormal, and reliable visual field tests. Second, several visual field tests were completed subsequent to the visual field POAG endpoint for most eyes, the number of these fields varying among eyes.
To address the issue of varying numbers of visual field tests per eye, we defined the outcome variable as the proportion of visual field tests subsequent to a visual field POAG endpoint that were normal. We applied the arc-sin-square root transformation to the outcome variable, a transformation commonly employed for comparing proportions in regression models.4,5 Results for proportion of visual field tests that were normal subsequent to a visual field POAG endpoint were obtained from a fit to a mixed model of the percentage of normal visual field tests, appropriately transformed, on the endpoint criteria, randomization assignment, baseline IOP (fixed effects) and patient (random effect). The random effect models the correlation between eyes from the same subject. The estimates and confidence intervals (CIs) were obtained using restricted maximum likelihood (REML) in Splus.6 A Cox proportional hazards model was additionally fit to study the effect of the strike rule on first reversion to a normal visual field following POAG onset. This model adjusted for the effects for randomization assignment, baseline IOP, and correlation between fellow eyes.
Results
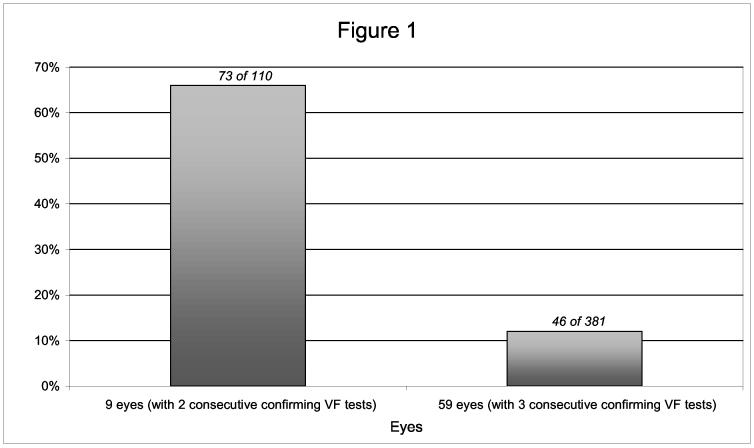
Sixty-eight eyes (from 64 patients) reached a POAG visual field endpoint as of 11/8/2001, with an average follow-up time from POAG endpoint to last follow-up visit of 35 months. Nine eyes (from 9 patients) reached a POAG visual field endpoint based on the confirmation criteria of two consecutive, abnormal, and reliable visual field tests with an average follow-up time from diagnosis of POAG to last follow-up visit of 59 months. These eyes had 110 follow-up visual field tests after reaching a POAG endpoint, 73 (66%) of which were normal. Fifty-nine eyes (from 56 patients) reached a POAG visual field endpoint based on the confirmation criteria of three consecutive, abnormal, and reliable visual field tests with an average follow-up time from diagnosis of POAG to last follow-up visit of 31 months. These eyes had 381 follow-up visual field tests after reaching a POAG endpoint, 46 (12%) of which were normal. The correlation between eyes from the same patient is significant at the 5% level (95% confidence interval on the random effect [(0.47, 2.38)] does not cover zero). As shown in Figure 1, the eyes reaching a visual field POAG endpoint based on the confirmation criteria of three consecutive, abnormal, and reliable visual field tests had significantly smaller percentage of normal follow-up visual field tests than those reaching endpoint based on the confirmation criteria of two consecutive, abnormal, and reliable visual field tests (P = 0.013).
Figure 1.
The percentage of normal follow-up VF tests following a VF POAG endpoint in nine eyes based on 2 consecutive, abnormal, and reliable VF tests and in fifty-nine eyes based on 3 consecutive, abnormal, and reliable VF tests. P = 0.013 Table 1: Frequency Distribution of Normal Visual Field Tests Following an OHTS Visual Field POAG Endpoint
As a confirmation of our results, we considered a secondary analysis which constrained the follow-up period, forcing similar follow-up intervals between the two groups: eyes reaching visual field POAG endpoint based on the confirmation criteria of three consecutive, abnormal, and reliable visual field tests and eyes reaching visual field POAG endpoint based on the confirmation criteria of two consecutive, abnormal, and reliable visual field tests. We limited the follow-up period for eyes under the two consecutive, abnormal, reliable visual field tests criteria to at most the median of the follow-up time of eyes under reaching POAG endpoint based on the confirmation criteria of three consecutive, abnormal, and reliable visual field tests.
Consequently in this analysis, both groups had a median follow-up time of 1135 days and a median of six follow-up visual field tests after POAG endpoint (thus an equal follow-up time interval and an equal number of follow-up visual field tests in the two groups). We fit a mixed model analogous to that put forth in the paper (adjusting for correlation between fellow eyes, treatment assignment, and IOP) and drew identical conclusions. Namely, the eyes reaching a visual field POAG endpoint based on the confirmation criteria of three consecutive, abnormal, and reliable visual field tests had a significantly smaller percentage of normal follow-up visual field tests (12%) than those reaching endpoint based on the confirmation criteria of two consecutive, abnormal, and reliable visual field tests (65%) (P = 0.033).
The frequency distribution of the percentage of subsequent normal follow-up visual field tests following an OHTS visual field POAG endpoint is shown in Table 1. Of the 9 eyes reaching an endpoint based on two consecutive, abnormal and reliable visual field tests, more than 76% of the subsequent follow-up field tests were normal for 4 of the eyes. Of the 59 eyes reaching endpoint based on three consecutive, abnormal and reliable visual field tests, less than 25% of the subsequent follow-up field tests were normal for 49 of the eyes.
Table 1.
Frequency Distribution of Normal Visual Field Tests Following an OHTS Visual Field POAG Endpoint
| Percentage of Subsequent Normal Field Tests After POAG Endpoint | Number of Eyes at Endpoint based on 2 consecutive, abnormal and reliable fields n = 9 eyes | Number of Eyes at Endpoint based on 3 consecutive, abnormal and reliable fields n = 59 eyes |
|---|---|---|
| 0 - 10% | 0 | 42 |
| 11 - 25% | 1 | 7 |
| 26 - 50% | 2 | 6 |
| 51 - 75% | 1 | 2 |
| 76 - 100% | 4 | 2 |
| (no fields performed) | 1 | 0 |
The odds of a reversion to a normal visual field, following POAG onset, is significantly larger for eyes reaching visual field POAG endpoint based on the confirmation of two consecutive, abnormal, and reliable visual field tests as compared to the confirmation of three consecutive, abnormal, and reliable visual field tests (p < 0.0001; odds ratio 5.70 with 95% CI 2.94 to 11.05). Furthermore, upon adjusting for the confirmation rule (2 vs 3 abnormal tests), treatment is not significantly related to reversion to a normal visual field (p = 0.10). This latter finding indicates that, since after POAG endpoint all eyes received treatment, the extended treatment received by the medication group does not have any significant beneficial effect on reversion to a normal visual field.
Discussion
In this report we compare the proportion of visual field tests that are normal subsequent to a visual field POAG endpoint confirmed by 2 vs. 3 consecutive, abnormal and reliable visual field tests with the defect in the same location. We found that 66% (73 of 110) of the subsequent visual field tests were normal in the group of 9 eyes that reached a visual field POAG endpoint based on two consecutive, abnormal, and reliable visual field tests. However, we found that only 12% (46 of 381) of the follow-up field tests were normal from the 59 eyes that reached a visual field POAG endpoint based on three consecutive, abnormal, and reliable visual field tests. Thus, an OHTS visual field POAG endpoint confirmed by three consecutive, abnormal, and reliable visual field tests appears to have greater specificity and stability than either one or two consecutive, abnormal and reliable visual field tests.
The OHTS protocol for determining the onset of POAG was designed to be highly specific to minimize clinical uncertainty as to who had developed POAG. The OHTS protocol included several provisions that protected the specificity of the diagnosis of POAG. The criteria for visual field abnormality were based on STATPAC. The protocol required that visual field abnormality be reproduced in the same location on separate, reliable tests completed at different visits. In June 1997, the visual field endpoint criterion was increased from 2 to 3 consecutive, abnormal and reliable visual field tests when it was found that 85.9% of the eyes with initial visual field abnormalities in OHTS had normal visual field tests upon repeat testing. In addition, 3 consecutive, abnormal and reliable visual field tests maintained a high specificity for determining POAG visual field endpoints. To protect against artifactual results, each abnormal visual field test was reviewed by masked trained readers at the Visual Field Reading Center. Visual field abnormalities that met the confirmation criteria were reviewed by the Endpoint Committee to insure that only those abnormalities specifically due to POAG were defined as POAG endpoints. The masked Endpoint Committee reviewed case report forms, visual field tests and optic disc photographs for both eyes from baseline in order to render an informed judgment as to whether the abnormality could be attributed to POAG. Only 125 (57%) of the 218 confirmed visual field abnormalities or optic disc deteriorations that were reviewed by the Endpoint Committee were attributed to POAG; 43% of the confirmed changes were due to causes other than POAG.1 Despite such thorough screening to protect the specificity of the determination of a visual field POAG endpoint, 12% of the eyes had one or more normal visual field tests subsequent to a visual field POAG endpoint even though confirmation required three consecutive abnormal and reliable visual field tests. These results suggest that either or both perimetric testing and early glaucomatous visual field loss may be inherently variable.
The follow-up interval differed between eyes in each of the two confirmation groups; POAG endpoint confirmed by two abnormal and reliable visual field tests and POAG endpoint confirmed by three abnormal and reliable visual field tests. In the analyses performed herein, we studied the percentage of normal visual field tests, being the number of normal tests divided by the total number of tests taken, thus overcoming difficulties presented by differences between follow-up intervals. This conclusion is further validated by a secondary analysis which forced an identical median follow-up interval and median number of follow-up visits between the two confirmation groups and drew identical conclusions.
The treatment differed in timing for the observation group, that is the time of treatment application after two versus after three consecutive, abnormal, and reliable visual field tests. For eyes assigned to the medication group, the confirmation rule effect is not compromised by such a timing difference as all eyes received treatment at baseline. In the observation or non-medication group, there is potential for a treatment timing effect as the eyes converting under the two consecutive, abnormal, and reliable visual field tests confirmation rule, on average, start treatment sooner (after only two consecutive, abnormal, and reliable visual field tests as opposed to three consecutive, abnormal, and reliable visual field tests) and thus may have a higher likelihood of normal visual field tests following POAG endpoint. This timing effect cannot be accounted for using the data from this study. Our best option, as put forth in the analysis herein, is to adjust the confirmation rule effect for treatment/randomization assignment. The confirmation rule effect is thus a real effect for eyes treated at baseline and indicative of a significant confirmation rule effect among eyes randomized to the observation group.
Sensitivity and Variability
Automated static perimetric threshold tests exhibit variability within a test procedure and from one examination to another, as reported in earlier investigations. The amount of variability is much higher in patients with glaucomatous visual field loss,7-16 especially at locations with reduced sensitivity. Previous studies have reported that increased visual field variability may be an early sign of glaucomatous damage.12 Hart and Becker demonstrated that glaucomatous visual field tests go through 3 transitional phases, which we have previously described.1 The initial phase has no defect demonstrable despite the fact that early damage is occurring. The second phase is a period in which shallow defects are often transient and are barely detectable. In the third phase, visual field defects progress at an uneven pace to become very dense. Variability in visual field results is unlikely to be due to poor reliability or poor testing procedure. A high quality of visual field data has been maintained because of strict quality control measures used by the OHTS Visual Field Reading Center (VFRC). The OHTS VFRC quality control system provides feedback on a regular basis to the clinical centers and to the visual field technicians about their performance and handling of the visual field data. In a prior presentation,17 the VFRC reported only 1,325 (2.6%) of the 50,925 regular follow-up visual field tests were beyond the 33% reliability limits for fixation losses, false positive errors, or false negative errors.
Long-Term Variability and Progression
It is difficult to distinguish between progression of glaucomatous visual field loss and long-term variability unless several visual field tests are obtained over time. Thus, it is necessary to confirm changes to avoid “overcalling” progressive visual field loss. For example, Shulzer found in the Low Tension Glaucoma Study that 4 to 6 confirming visual field tests were needed to reliably determine visual field progression.13 Chauhan and colleagues defined progression as at least 4 non-edge test points beyond the 5% probability level on the Glaucoma Change Probability (GCP) program, with complete overlap of at least 4 of these points on a confirming field.14 Although similar strategies for determining visual field progression were used in the Early Manifest Glaucoma Trial, the Glaucoma Change Probability program was based on the Pattern Deviation values rather than the Total Deviation values. In addition, three test points beyond the 5% level were needed for confirmation of progression on 3 successive visual field tests.
The Advanced Glaucoma Intervention Study (AGIS) visual field defect score is based on both the extent and depth of clusters of adjacent depressed test locations relative to age-matched normative data for individuals tested with the Humphrey Visual Field Analyzer 24-2 full-threshold testing program. The complex AGIS scoring system was designed for use with more advanced glaucomatous visual field loss and has been described in detail elsewhere.18,19 In the AGIS study, only one baseline visual field was obtained and three consecutive field tests showed worsening of 4 AGIS units indicated progression. During follow up, in a subset in which the IOP was always less than 18 mm Hg and there was no net visual field progression in 8 years, 14% of the subjects had an improvement of 4 AGIS units and 14% worsened at 5 years of follow up.20 The Collaborative Initial Glaucoma Treatment Study (CIGTS) scoring system21 has also been described elsewhere and is based on the total deviation data from a HFA 24-2 full-threshold testing program but differs because it is based on probability rather than the depth of the defect.22,23 At 5 years of follow up in CIGTS, no net visual field progression was noted in either the medicine first or surgery first arms. About 10% of the subjects met the criterion for progression at any time point. The percentage improving by the same criterion was not reported. Comparison of AGIS and CIGTS scoring systems using a common longitudinal data set have shown that the CIGTS system identifies progression twice as frequently as the AGIS system.24,25
An alternative to using specified endpoint changes on a specified number of consecutive fields is to use a linear regression analysis. The length of follow-up required to detect progression using linear regression is influenced by a number of factors, including examination frequency, underlying rate and type of progression, the specific variable being evaluated, the degree of variability, and order of the visual field tests within the time series.26 Spry, Johnson, and Vesti,27,28 reported that a minimum of 7 or 8 visual field tests are required to achieve reasonable levels of sensitivity and specificity.29,30
Confirmation of visual field change at successive examinations and correlation of visual field changes with other clinical observations (optic disc change, nerve fiber layer change, etc.) seems to be the best method of detection of progression31-40.
Specificity
It is critical to have a high specificity to evaluate the treatment effect in clinical trials. It is also important to have high specificity when diagnosing early POAG. Before a clinician commits an individual to a lifetime of medical treatment, he/she should have a high degree of certainty about the diagnosis. The clinician has the ability to evaluate other clinical parameters, such as optic nerve head measurements and intraocular pressures. Thus, the practitioner may not need three consecutive abnormal visual field tests to confirm a glaucomatous visual field abnormality if an optic disc hemorrhage or progressive cupping is present. However, if the optic nerve findings are equivocal, the criterion of three consecutive abnormal and reliable visual field tests may be useful before instituting a lifetime of glaucoma treatment.
The clinical significance of a sporadic abnormal visual field test is not yet clear. It is possible that such a field is an indication of early glaucomatous damage. It is also possible that a dose-response relationship exists so that an individual with two abnormal visual field tests over five years of testing may be more likely to develop permanent glaucomatous damage than an individual with one abnormal field test over five years of testing. Further follow-up of the OHTS participants may shed light on this question. Currently, we can conclude that confirmation of visual field abnormalities on at least three visual field tests helps to distinguish between the development of glaucomatous visual field loss and long-term variability.
Footnotes
Supported in part by: Grants EY 09307 and EY 09341 from the National Eye Institute and the National Center on Minority Health and Health Disparities, National Institutes of Health, Bethesda, MD; Merck Research Laboratories, White House Station, NJ; and by an unrestricted grant from Research to Prevent Blindness, Inc, New York, NY.
References
- 1.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study. a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 2.Keltner JL, Johnson CA, Quigg JM, et al. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 3.Keltner JL, Johnson CA, Cello KE, et al. Classification of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2003;121:643–650. doi: 10.1001/archopht.121.5.643. [DOI] [PubMed] [Google Scholar]
- 4.Draper NR, Smith H. Applied Regression Analysis. Wiley; New York: 1981. p. 239. [Google Scholar]
- 5.Cook RD, Weisberg S. Applied Regression Including Computing and Graphics. Wiley; New York: 1999. p. 318. [Google Scholar]
- 6.Pinheiro JC, Bates DM. “Fitting Linear Mixed-Effects Models,” Mixed-Effects Models in S and S-PLUS. Springer; New York: 2000. [Google Scholar]
- 7.Holmin C, Krakau CE. Variability of glaucomatous visual field defects in computerized perimetry. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1979;210:235–250. [PubMed] [Google Scholar]
- 8.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989;108:130–135. doi: 10.1016/0002-9394(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan BC, Tompkins JD, LeBlanc RP, et al. Characteristics of frequency-of-seeing curves in normal subjects, patients with suspected glaucoma, and patients with glaucoma. Invest Ophthalmol Vis Sci. 1993;34:3534–3540. [PubMed] [Google Scholar]
- 10.Wall M, Maw RJ, Stanek KE, et al. The psychometric function and reaction times of automated perimetry in normal and abnormal areas of the visual field in patients with glaucoma. Invest Ophthalmol Vis Sci. 1996;37:878–885. [PubMed] [Google Scholar]
- 11.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–1549. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- 12.Hart WM, Becker B. The onset and evolution of glaucomatous visual field defects. Ophthalmology. 1982;89:268–279. doi: 10.1016/s0161-6420(82)34798-3. [DOI] [PubMed] [Google Scholar]
- 13.Schulzer M. Normal tension Glaucoma Study Group. Errors in the diagnosis of visual field progression in normal tension glaucoma. Ophthalmology. 1994;101:1589–1594. doi: 10.1016/s0161-6420(94)31133-x. [DOI] [PubMed] [Google Scholar]
- 14.Chauhan BC, House PH, McCormick TA, et al. Comparison of conventional and high pass resolution perimetry in a prospective study of patients with glaucoma and healthy controls. Arch Ophthalmol. 1999;117:24–33. doi: 10.1001/archopht.117.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Werner EB, Petrig B, Krupin T, et al. Variability of automated visual fields in clinically stable glaucoma patients. Invest Ophthalmol Vis Sci. 1989;30:1083–1089. [PubMed] [Google Scholar]
- 16.Boeglin RJ, Caprioli J, Zulauf M. Long-term fluctuation of the visual field in glaucoma. Am J Ophthalmol. 1992;113:396–400. doi: 10.1016/s0002-9394(14)76161-6. [DOI] [PubMed] [Google Scholar]
- 17.Keltner JL, Johnson CA, Cello KE, et al. Visual field technician performance in the Ocular Hypertension Treatment Study (OHTS) Invest Opthalmol Vis Sci. 2001;42(4):S152. [Google Scholar]
- 18.AGIS investigators: Advanced Glaucoma Intervention Study 2 Visual field test scoring and reliability. Ophthalmology. 1994;101:1445–1455. [PubMed] [Google Scholar]
- 19.AGIS investigators: The Advanced Glaucoma Intervention Study (AGIS) 1. Study design and methods and baseline characteristics of study patients. Controlled Clinical Trials. 1994;15:299–325. doi: 10.1016/0197-2456(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 20.AGIS investigators: The Advanced Glaucoma Intervention Study (AGIS) 7. the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 21.Musch DC, Lichter PR, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–662. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 22.Heijl A, Lindgren G, Olsson J, et al. Visual field interpretation with empiric probability maps. Arch Ophthalmol. 1989;107:204–208. doi: 10.1001/archopht.1989.01070010210024. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz B, Nagin P. Probability maps for evaluating automated visual fields. Doc Ophthalmol Proc Ser. 1984;42:39–48. [Google Scholar]
- 24.Katz J. Scoring systems for measuring progression of visual filed loss in clinical trials of glaucoma treatment. Ophthalmology. 1999;106:391–395. doi: 10.1016/S0161-6420(99)90052-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee AC, Sample PA, Blumenthal EZ, et al. Comparison of visual field progression algorithms. Invest Ophthalmol Vis Sci. 1999;40(Supplement):S68. [Google Scholar]
- 26.Wild JM, Hutchings N, Hussey MK, et al. Pointwise univariate linear regression of perimetric sensitivity against follow-up time in glaucoma. Ophthalmology. 1997;104:808–815. doi: 10.1016/s0161-6420(97)30229-2. [DOI] [PubMed] [Google Scholar]
- 27.Spry PG, Johnson CA. Identification of Progressive Glaucomatous Visual Field Loss. Surv Ophthalmology. 2002;47:158–173. doi: 10.1016/s0039-6257(01)00299-5. [DOI] [PubMed] [Google Scholar]
- 28.Vesti E, Johnson CA, Chauhan BC. Comparison of different methods for detecting glaucomatous visual field progression. Invest Ophthalmol Vis Sci. 2003;44(9):3873–3879. doi: 10.1167/iovs.02-1171. [DOI] [PubMed] [Google Scholar]
- 29.Holmin C, Krakau CE. Regression analysis of the central visual field in chronic glaucoma cases. A follow-up study using automatic perimetry. Acta Ophthalmol (Copenh) 1982;60:267–274. doi: 10.1111/j.1755-3768.1982.tb08381.x. [DOI] [PubMed] [Google Scholar]
- 30.Spry PG, Bates AB, Johnson CA, et al. Simulation of longitudinal threshold visual field data. Invest Ophthalmol Vis Sci. 2000;41:2192–2200. [PubMed] [Google Scholar]
- 31.Birch MK, Wishart PK, O’Donnell NP. Determining progressive visual field loss in serial Humphrey visual fields. Ophthalmology. 1995;102:1227–1234. doi: 10.1016/s0161-6420(95)30885-8. [DOI] [PubMed] [Google Scholar]
- 32.Smith SD, Katz J, Quigley HA. Analysis of progressive change in automated fields in glaucoma. Invest Ophthalmol Vis Sci. 1996;37:1419–1428. [PubMed] [Google Scholar]
- 33.Mikelberg FS, Drance SM. The mode of progression of visual field defects in glaucoma. Am J Ophthalmol. 1984;98:443–445. doi: 10.1016/0002-9394(84)90128-4. [DOI] [PubMed] [Google Scholar]
- 34.Mikelberg FS, Schulzer M, Drance SM, Lau W. The rate of progression of scotomas in glaucoma. Am J Ophthalmol. 1986;101:1–6. doi: 10.1016/0002-9394(86)90457-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee AC, Sample PA, Blumenthal EZ, et al. Infrequent confirmation of visual field progression. Ophthalmology. 2002;109:1059–1065. doi: 10.1016/s0161-6420(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 36.Nouri-Mahdavi K, Brigatti L, Weitzman M, et al. Comparison of method to detect visual field progression in glaucoma. Ophthalmology. 1997;104:1228–1236. doi: 10.1016/s0161-6420(97)30153-5. [DOI] [PubMed] [Google Scholar]
- 37.Rasker MT, van den Enden A, Bakker D, et al. Rate of visual field loss in progressive glaucoma. Arch Ophthalmol. 2000;118:481–488. doi: 10.1001/archopht.118.4.481. [DOI] [PubMed] [Google Scholar]
- 38.Drance SM, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131(6):699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 39.Kwon YH, Chang-Sik K, Zimmerman BM, et al. Rate of visual field loss and long-term visual outcome in primary open-angle glaucoma. Am J Ophthalmol. 2001;132:47–56. doi: 10.1016/s0002-9394(01)00912-6. [DOI] [PubMed] [Google Scholar]
- 40.Chen PP. Correlation of visual field progression between eyes in patients with open-angle glaucoma. Ophthalmology. 2002;109:2093–2099. doi: 10.1016/s0161-6420(02)01241-1. [DOI] [PubMed] [Google Scholar]