Abstract
Alteration of apoptotic activity has been observed in a number of tissues in aging mammals, but it remains unclear whether and/or how apoptosis may affect aging. Caspase-2 is a member of the cysteine protease family that plays a critical role in apoptosis. To understand the impact of compromised apoptosis function on mammalian aging, we conducted a comparative study on caspase-2 deficient mice and their wild-type littermates with a specific focus on the aging-related traits at advanced ages. We found that caspase-2 deficiency enhanced a number of traits commonly seen in premature aging animals. Loss of caspase-2 was associated with shortened maximum lifespan, impaired hair growth, increased bone loss, and reduced body fat content. In addition, we found that the livers of caspase-2 deficient mice had higher levels of oxidized proteins than those of age-matched wild-type mice, suggesting that caspase-2 deficiency compromised the animal's ability to clear oxidatively damaged cells. Collectively, these results suggest that caspase-2 deficiency affects aging in the mice. This study thus demonstrates for the first time that disruption of a key apoptotic gene has a significant impact on aging.
Keywords: caspase-2, maximum lifespan, bone, hair growth, fat
Introduction
Apoptosis is a genetically programmed mechanism that control cell death (Zhang et al., 2002a). Two well-documented apoptotic paradigms are the extrinsic and intrinsic pathways of apoptosis. The extrinsic pathway, exemplified by Fas ligand-induced apoptosis, is achieved by sequential activation of initiator and executioner caspases without the involvement of mitochondria. The intrinsic pathway is mediated by mitochondria, which propagate apoptotic signals by releasing the proapoptotic factors such as cytochrome c that activate downstream executioner caspases.
Apoptosis is implicated in a number of diseases such as neuronal degenerative diseases that are prevalent in the elderly (Zhang et al., 2002a). A number of studies have found age-dependent changes of apoptotic cell death in various tissues in rodents (Taglialatela et al., 1996; Fujino et al., 1996; Adams et al., 1998; Muskhelishvili et al., 1995; Higami et al, 1997). In addition, the activity of caspases, the key mediator of apoptosis, was found to be increased in multiple organs in old rodents (Zhang et al., 2002b). At present, it remains unclear whether and/or how apoptosis may affect aging.
Addressing this issue requires the utilization of animal models with genetically modified apoptotic function. Finding or generating such animals has proven to be very challenging. Because apoptosis is critical for many essential biological functions such as embryonic development, immunity, maintenance of tissue homeostasis and defense against tumorigenesis, a significant and wide-spectral disruption of apoptotic function often leads to severe organ defects or health problems (Zhang et al., 2002a). As a result, these animals often die either prenatally or at young ages, making them unavailable for aging studies.
In the search for an animal model for this study, we were attracted by caspase-2 knockout mice. The caspase-2 knockout mouse has several interesting features that make it a very promising model for the study of the role of apoptosis in aging. First, caspase-2 has been found to mediate apoptosis in many different types of cells (Troy et al., 2003). Second, caspase-2 is activated in response to a wide range of physiological signals, including oxidative stress that is considered to be a major factor of aging (Lopez-Cruzan et al., 2005; Prasad et al., 2006). Thirdly, caspase-2 knockout mice appeared to develop and mature normally without major health problems (at least at young ages according to the literature)(Bergeron et al., 1998), which may allow the opportunity for caspase-2 knockout mice to live long enough for the examination of the effect of apoptosis deficiency on aging-related traits that show up only at advanced ages. Therefore, we chose to use the caspase-2 knockout mouse as a model to study the role of apoptosis in aging. Here, we summarize our findings including the observation that loss of caspase-2 function enhances multiple aging-related traits that are commonly seen in premature aging animals.
Materials and Methods
Animals
The caspase-2 knockout mice were originally generated by Junying Yuan and kindly provided by Dr. Carol Troy of Columbia University with Dr. Yuan's consent (Bergeron et al., 1998). The deleted fragment comprised the exon that encodes the QACRG active site of the enzyme and part of the next exon that encodes the caspase-2 short isoform. The deletion thus inactivates both the long and short form of caspase-2. The mice were backcrossed with C57Bl/6 once in our animal facility and used as founders. Caspase-2 knockout and positive animals used in the experiments are littermates.
Quantification of BMD and body fat
Mice were sacrificed by CO2 inhalation. Measurements of BMD and fat content were obtained by duel energy X-ray absorptiometry (DEXA) scan using a Lunar PIXImus imager (GE Medical Systems). Fat content (percentage) and BMD were determined using whole body scans of the mice.
99mTc-MDP Micro- SPECT/CT (Yildirim et al., 2004)
Conscious mice were injected with 1mCi 99mTc-MDP (GE Healthcare Radiopharmacy, San Antonio, TX) in 0.2 ml saline via the tail vein. At 3 hours post-injection, animals were anesthetized with ketamine intraperitoneally and placed in prone position on the bed of the XSPECT imager (Gamma Medica, Northridge, CA). A SPECT image was acquired using 32 projections with the dual gamma cameras (parallel-hole collimators) moving in 180° rotation. To allow for anatomical co-registration, the SPECT image was followed by a CT image acquisition (X-ray source: 50 kVp, 600 mA; 256 projections) while precisely maintaining the position of the animal. The software provided with the XSPECT imager was used for the SPECT and CT image reconstruction and the SPECT/CT image fusion. SPECT images were reconstructed taking into account the radius of rotation to produce image sizes of 56×56×56. The CT images were also reconstructed resulting in image sizes of 512×512×512 with a 0.15 mm image resolution. Anterior and posterior whole body 5 min planar images were also acquired using parallel hole collimators and with a standard (1/100 of injected dose) in the field of view. The percentage of activity in the whole body was calculated from the planar images created by drawing regions of interest using the standard source as a point of reference. Endpoint biodistribution studies were performed at sacrifice immediately following Micro-SPECT imaging. Tissues were harvested and placed in individual scintillation vials containing 10% buffered formalin. The uptake of 99mTc-MDP in each tissue was assessed using a Wallac 1480 automated gamma well counter (Perkin Elmer Life Sciences, Boston, MA).
Histology and histomorphometry (Boyce et al., 1992)
At sacrifice, tissues were harvested and immediately placed in 10% buffered formalin. Bones were decalcified in 10% ethylenediaminetetraacetic acid. Tissues were embedded in paraffin, sectioned and stained with haematoxylin and eosin for measurements of trabecular bone volume. Serial sections were tartrate resistant acid phosphatase (TRAP)-stained for counting of osteoclasts. Quantitative detailed bone histomorphometry was performed on sections of the spine from Caspase-2 knockout and wild-type mice using Osteomeasure (OsteoMetrics, Decatur, GA).
Measurement of global levels of irreversible oxidation of cysteine residues in proteome
Mice liver proteins were homogenized in buffer (50 mM phosphate buffer pH 7.9, 0.5 mM MgCl2, 1 mM ethylenediaminetetraacetic acid and cocktail of protease inhibitors) and centrifuged at 100,000g at 4°C in the dark for 1 hr. Proteins in the supernatant (cytosolic) were treated with urea (6M) and dithiothreitol (2mM) for 1 hr in the dark at 37°C followed by the treatment with 6-iodoacetamidofluorescein (6-IAF) (10mM) and incubation for another 45 min at 37°C in the dark. The proteins were then precipitated with equal volumes of 20% trichloroacetic acid and centrifuged at 16000g for 20 min. The pellets were washed extensively with ethanol/ethyl acetate (1:1) (v/v) to remove excess 6-IAF. 6-IAF labeled protein pellets were then finally dissolved in phosphate buffer (pH 7.9) containing urea (6M) and the protein concentrations were measured by Bradford reagent. Equal amounts of proteins from different experimental aliquots were then run in 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis to resolve the fluorescence-labeled proteins. After electrophoresis, the image of the fluorescent protein for each lane on the gel was captured with Typhoon 9400 (emission filter 526 nm) (Amersham Biosciences, Piscataway, NJ). The same gel was then stained with Coomassie blue staining reagent for 2 hr, and the image of the Coomassie-stained proteins in each lane of the gel was captured with Alphaimager 3400 (Alpha Innotech, San Leandro, CA). The intensities of fluorescence and the Coomassie blue stain were calculated using ImageQuant 5.0 software (Amersham Biosciences) for each lane of the gel and the data were expressed in the ratio of fluorescence (F) and Coomassie blue stain (C).
Statistical analysis
All the data were analyzed with GraphPad Prism program (GraphPad, Inc. CA). One way ANOVA or unpaired t-test was used when appropriate. P<0.05 is considered to be significant.
Results
Lifespan reflects an animal's overall health and the rate of aging. As shown in Figure 1A, caspase-2 knockout mice had almost the same median lifespan (950 days) as the wild-type littermates (954 days). However, from that time point on, the survival curve of caspase-2 knockout mice diverged from that of the wild-type mice (Figure 1B). There was a small difference in the mean lifespan between caspase-2 knockout mice (903 days) and the wild-type littermates (935 days). However, there was a large and significant difference in the maximum lifespan (mean of the last 10 percentile of the mice) between caspase-2 knockout mice and the wild-type littermates. The maximum lifespan of caspase-2 knockout mice was 117 days (∼4 months or 10%) shorter than that of the wild-type mice (1137 versus 1254 days). None of the caspase-2 knockout mice lived beyond 1200 days, whereas 12.5% of wild-type littermates did. Log-rank test of the last 50 percentile of animals (i.e., animals living longer than the group's median lifespan) showed that caspase-2 knockout mice lived statistically shorter (p<0.01) than the wild-type littermates.
Figure. 1.
Lifespan A. Survival curves for all mice (n=64 for each group). B. Survival curves for last 50 percentile mice in each cohort (n=32 for each group).
Apoptosis serves as an endogenous defense mechanism against tumorigenesis. It is a widely-held view that defects in apoptosis may lead to increased tumorigenesis (Dlamini et al., 2005). Therefore, our initial hypothesis was that caspase-2 deficiency might lead to increased tumorigenesis. However, after a comprehensive examination of various tumors in the mice at 24-26 months of age, we found that caspase-2 deficiency did not increase tumor incidence (Table 1). The overall tumor burden (the sum of different types of tumors in a mouse) was 0.44 for caspase-2 knockout mice, 0.66 for the wild-type mice, and 0.61 for the heterozygous mice. The percentage of tumor bearing mice (the percentage of mice that have one or more neoplastic lesions in an experimental group) was 39.3% for caspase-2 knockout mice, 55.2% for the wild-type mice and 48.5% for heterozygous mice. We also examined the incidence of a number of non-neoplastic pathologies/diseases including glomerulonephritis, lymphocyte infiltration, hydronephrosis, acidophilic macrophage pneumonia, brain psammoma body, angiectasia and arteritis in the mice (Table 1). Caspase-2 deficiency did not increase the incidence of these pathologies/diseases, either. Therefore, the shortened lifespan of caspase-2 knockout mice was not caused by increased tumor incidence or these non-neoplastic pathologies/diseases.
Table 1.
Comparative pathology of old mice (24-26 months of age)
| Caspase-2(+/+) n=29 |
Caspase-2(+/−) n=33 |
Caspase-2(−/−) n=28 |
|
|---|---|---|---|
| Neoplastic lesions | |||
| Lymphoma | 10 | 8 | 4 |
| Lung carcinoma | 6 | 3 | 2 |
| Hepatocellular carcinoma | 1 | 1 | 1 |
| Hemangioma | 2 | 7 | 5 |
| Pheochromocytoma | 0 | 1 | 0 |
| Tumor burden | 0.66 | 0.61 | 0.44 |
| Number of tumor bearing mice | 16(55.2%) | 16(48.5%) | 11(39.3%) |
| Non-neoplastic lesions | |||
| Glomerulonephritis | 26 | 26 | 19 |
| Lymphocyte infiltration | 24 | 23 | 17 |
| Hydronephrosis | 1 | 2 | 2 |
| Acidophilic Macrophage Pn. | 4 | 1 | 1 |
| Psammoma body (brain) | 9 | 9 | 11 |
| EMH (spleen) | 9 | 13 | 2 |
| Angiectasia | 1 | 0 | 1 |
| Arteritis | 1 | 1 | 0 |
Based on histopathology data collected from each mouse, the percentage of tumor-bearing mice, tumor burden and incidence of disease in each group were calculated. The percentage of tumor-bearing mice was calculated as the percentage of mice in an experimental group that had one or more neoplastic lesions. The tumor burden was calculated as the sum of different types of tumor in a mouse.
Since the reduction of the survival rate in caspase-2 knockout mice began only at a later stage of life and the pathological study did not find the cause for the shortened lifespan of caspase-2 knockout mice, we asked whether the shortened lifespan of caspase-2 knockout mice may be due to increased rate of aging. Because there is no single definitive biological marker of aging, the change of aging rate is always assessed by examining the animal's lifespan together with a number of aging-dependent traits, most important of which are skeletal health, hair growth and subcutaneous fat content (Tyner et al., 2002; Trifunovic et al., 2004).
Mammalian bone is a dynamic tissue that is continually remodeled throughout life (Blair et al., 1993). Through this remodeling, localized new bone formation by osteoblasts replaces bone that is resorbed by osteoclasts. One of the hallmarks of aging is a progressive loss of bone mass due to an increase in bone resorption activity relative to bone formation activity.
A number of researchers have reported that several premature aging mice had severe kyphosis (Tyner et al., 2002; Trifunovic et al., 2004). In their papers, the evidence was demonstrated by representative images, but not by quantitative measurement of the severity of the kyphosis. In fact, there is no accepted or consensus method for the measurement of the degree of kyphosis in mice. We tried a method described by Garcia-Cao et al. (2002) and found it too subjective. Therefore, we chose not to use kyphosis as an indicator of aging-dependent skeletal change. The bone mass can be accurately measured. We examined bone mineral density (BMD) of the mice using DEXA scan. At 14 months of age, the BMD of caspase-2 knockout mice was not significantly different from that of wild-type littermates (Figure 2E). However, at the age of 24-26 months, caspase-2 knockout mice had a significantly lower BMD than wild-type mice (Figure 2F). To further investigate this phenotype, we performed quantitative histomorphometric analysis of the vertebral bodies of old mice (29 months of age) and found that caspase-2 knockout mice exhibited decreased trabecular bone (indicated by arrows in Figure 2A and 2B) volume compared to age-matched wild-type mice (Figure 2G). We counted the number of osteoblasts and osteoclasts per mm of the bone surface. Aged caspase-2 knockout mice had similar numbers of osteoblasts (Figure 2H), but increased numbers of osteoclasts (indicated by arrows in Figure 2C and 2D) compared to the wild-type mice (Figure 2I). These results indicate that caspase-2 knockout mice had a more severe aging-dependent bone loss at advanced ages. Furthermore, these results also suggest that the mechanism of increased bone loss in caspase-2 knockout mice may be due to enhanced bone resorption. In vivo rates of bone resorption can also be determined by measuring the products of bone resorption in urine. Type I collagen, which accounts for approximately 90% of the organic matrix of bone, is cross-linked by specific molecules such as deoxypyridinoline (DPD). Resorption of the bone causes release of DPD into the circulation and excretion in urine (Delmas et al., 1991). Urinary DPD levels normalized against urinary creatinine concentrations reflect rates of bone resorption in vivo. As shown in Figure 3J, at 26 months of age, caspase-2 knockout mice had much higher rates of bone resorption than wild-type littermates based upon the level of DPD urinary excretion. These results demonstrated that caspase-2 deficiency enhances aging-dependent bone loss at least partially through enhancing bone resorption.
Figure 2.
Skeletal traits. A. Section of vertebral body of old wild-type mice (29 months of age) stained by haematoxylin and eosin. B. Section of vertebral body of old caspase-2 knockout mice stained by haematoxylin and eosin. Arrows in A and B indicate trabecular bones. C. Tartrate resistant acid phosphatase (TRAP) staining of vertebral body section of old wild-type mice. D. TRAP staining of vertebral body section of old caspase-2 knockout mice. Arrows in C and D indicate osteoclasts. E. Whole body BMD in 14 months old mice (n=5 for each group). F. Whole body BMD in old mice (n=28-34). G. trabecular bone volume (BV) versus total volume of vertebral marrow cavity in old mice (n=3-4). H. Osteoblast number on the bone surface. I. Osteoclast number. J. Urinary deoxypyridonoline concentrations normalized against creatinine (n=9-11; age=26-28-months) * indicates p<0.05. Error bars represent SEM.
Figure 3.
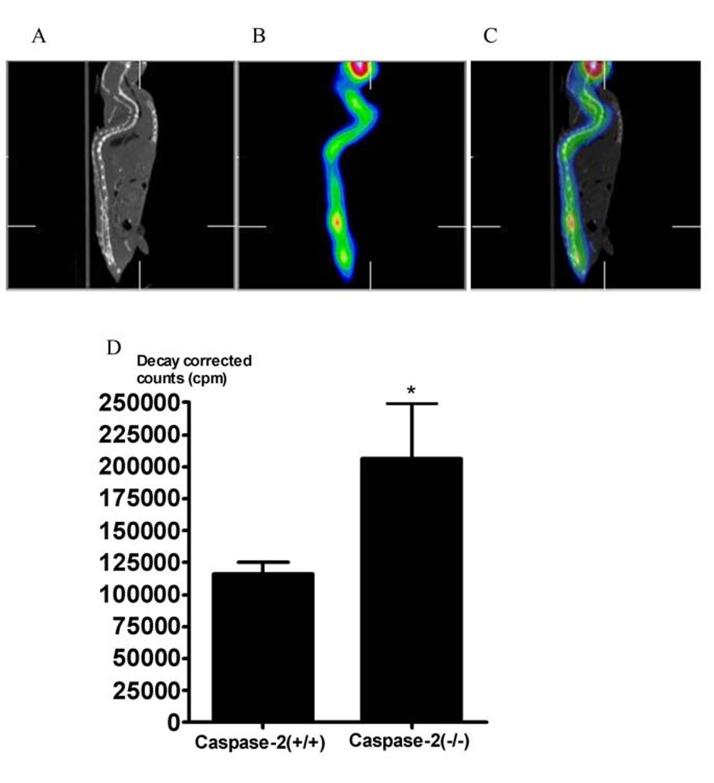
Skeletal uptake of99mTc-MDP. A. The representative micro-CT image of a wild-type mouse at the age of 29 months. B. The distribution of 99mTc-MDP in skeleton in the same mouse as detected by micro-SPECT . C. The overlay of the left and middle panel. D. The region of interest analysis for the whole body count of 99mTc-MDP in old mice (n=5). Error bars represent SEM.
Technetium −99m methylene diphosphonate (99mTc-MDP) is a widely used bone-scanning agent (Subramanian et al., 1975). Because the diphosphonate group has a very high affinity for the inorganic matrix of the bone, 99mTc-MDP is concentrated mainly in the skeletal system. The uptake of 99mTc-MDP is particularly high at active bone remodeling sites because more bone mineral surfaces are exposed by osteoclasts. Clinically, it has been found that the total skeletal uptake of 99mTc-MDP is increased in osteoporotic patients due to increased bone turnover activity (Carnevale et al., 2000). We used micro-single photon emission computed tomography (SPECT) to compare 99mTc-MDP uptake in male caspase-2 knockout and wild-type mice at the age of 29 months Figure 3A shows the micro-computed tomography (CT) image of a mouse. Figure 3B shows the distribution of 99mTc-MDP in the body as detected by micro-SPECT. Figure 3C is the overlay of Figures 3A and 3B that shows the concentration of 99mTc-MDP in the skeleton. Quantitative measurement revealed that caspase-2 knockout mice had a significantly higher skeletal uptake of 99mTc MDP than the wild-type mice (Figure 3D). Therefore, there results are consistent with those of bone densitometry and histomorphometry.
Hair growth declines as a function of age in mice (Harrison et al., 1988). Hair regrowth ability has been widely used as a measurement of aging (Tyner et al., 2002; Trifunovic et al., 2004). To measure the hair growth ability, we shaved a dorsal segment (2×3 cm) of skin on the mouse. After 30 days, the number of mice whose hair failed to grow back was counted. The number is 40% (6 out of 15) for old caspase-2 knockout mice (24 months of age) and 10% (1 out of 10) for age-matched wild-type mice. After 48 days, all wild-type mice had the hair grown back (n=10), but there were still 21.4% (3 out of 14) of caspase-2 knockout mice whose hair failed to grow back. Therefore, the hair growth ability in old caspase-2 knockout mice is impaired relative to that in age-matched caspase-wild type mice.
Loss of body fat, especially subcutaneous fat, and body weight, is common in very old people. Due to uneven distribution, it is very difficult, if not impossible, to accurately quantify the subcutaneous fat content. As an alternative way, we measured the whole body fat content with DEXA scan and found that caspase-2 knockout mice at advanced ages had a lower body fat content than age-matched wild-type littermates (Figure 4).
Figure 4.
Body fat content. Body fat content of the mice at the age of 24-26 months measured by DEXA scan (n=28-34).
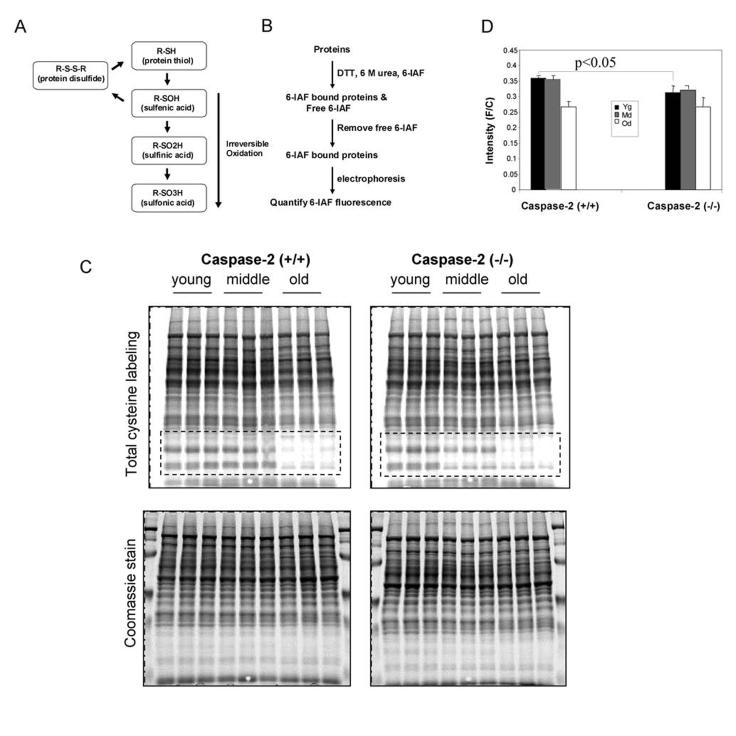
Caspase-2 is activated by oxidative stress and mediates oxidative stress-induced cell death (Lopez-Cruzan et al., 2005; Prasad et al., 2006). As a consequence of loosing caspase-2 function, the animals may not be able to efficiently clear oxidatively damaged cells. Liver is where a lot of oxidative reaction occurs. We hypothesized that if the oxidatively damaged cells are not promptly cleared through apoptosis, this would result in increased levels of oxidized macromolecules in the liver. Therefore, we examined the extent of global protein oxidation in the liver. The amount of cysteine residues that have not been irreversibly oxidized is inversely proportional to the cellular content of oxidized protein (Figure. 5A). We used the assay described in Figure 5B to measure the cysteine oxidation in the liver of caspase-2 knockout and wild-type mice (Chaudhuri et al., 2001). Cysteine residues on proteins were first exposed by treating proteins with dithiothreitol and 6M urea, and then labeled with 6-iodoacetamidofluorescein (6-IAF). After the removal of free 6-IAF, the 6-IAF-labeled proteins were separated on a polyacrylamide gel and their amount was quantified. A low amount of 6-IAF-labelled proteins corresponds to a high degree of irreversible cysteine oxidation. There was a general trend of an increase of protein oxidation with an increase in animal's age, which can be seen clearly in the boxed areas in Figure 5C and in Figure 5D. This is consistent with other studies (Bokov et al., 2004). Interestingly, we found that caspase-2 knockout mice had a higher level of protein oxidation than the wild type littermates at 6 months of age (Figure 5D), which suggests that there are more oxidatively damaged cells in the caspase-2 knockout mice due to deficiency of caspase-2-mediated apoptosis.
Figure 5.
Cysteine oxidation in caspase-2 deficient and wild-type mice. A, Schematic representation of different states of oxidation of cysteine residues in proteins. B. Schematic representation of cysteine oxidation assay. C. Global measurement of irreversible oxidation of cysteine residues in liver proteins. Liver cytosolic proteins were isolated from young (5 months of age), middle (14 months of age) and old (27 months of age) wild-type and caspase-2(−/−) mice and labeled with 6-iodoacetamidofluorescein (6-IAF) as described in Method section. Upper panel photographs show the fluorescence (F) of the gel as a measure of 6-IAF binding to the protein extracts isolated from the young, middle and old wild-type and caspase 2(−/−) mice. Lower panel photographs show the gels stained with Coomassie staining to measure the protein content (C) loaded in each lane of the gel. The boxed areas in the photographs show the dramatic differences between young and old and between wild-type and caspase-2 (−/−) samples. D. Graphical representation of the ratio of fluorescence (F) and Coomassie staining (C) was derived from B. The data are presented as the mean and SEM of data from three mice for each age group. Values obtained young caspase-2 (−/−) mice are significantly lower (P<0.05) than the values obtained from young wild-type mice.
Discussion
Whether and/or how apoptosis may affect aging is a very interesting but unanswered question. Theoretically, an abnormal increase of apoptosis activity may cause excessive loss of cells essential for normal physiological functions, leading to acceleration of aging. However, an abnormal decrease of apoptosis activity may also accelerate aging due to extended survival of certain types of cells whose functions contribute to aging or aging-related traits. The research on this issue has been hampered by the lack of appropriate animal models. Fortunately, caspase-2 knockout mouse turns out to be a very good model for the study of the effect of apoptosis on aging.
Although there is not a single definitive biological marker that can be used quantify the rate of aging, the change of aging rate in mice can be revealed by quantitative measurement of a group of aging-related traits. Lifespan is unquestionably the most important trait reflective of aging rate. The most reasonable explanation for the shortened lifespan of caspase-2 knockout mice is aging because caspase-2 knockout mice had similar incidence of aging-unrelated pathologies/diseases compared to the wild-type littermates (Table 1) and the survival rate of caspase-2 knockout and wild type diverge in an aging-dependent way, i.e., only at advanced ages when the effect of aging on animal's survival began to show up. In contrast to mean lifespan, which is significantly affected by disease and non-biological factors, maximum lifespan is determined by “rate of aging”. For example, in ancient Rome the mean lifespan for humans was <30 years. Today, people in the most developed countries have a mean lifespan of >70 years. However, despite the steady improvement of mean lifespan, maximum lifespan for humans has remained about 115–120 all through known history (Leonid et al., 1991). Therefore, a shortened maximum lifespan indicates that caspase-2 deficiency accelerated aging in the mice.
During the lifespan of mammals, bone mass changes as a result of changes in the ratio of bone formation activity and bone resorption activity (Blair et al., 1993). From childhood to adulthood, the bone formation activity is greater than bone resorption activity, which results in a net increase of bone mass. At advanced ages, the balance tips towards bone resorption, resulting in a net loss of bone mass. This makes bone mass a valuable marker reflective of aging (Rico et al., 1995; Tyner et al., 2002; Trifunovic et al., 2004). Although other factors may also affect bone mass, the case is strong that the bone loss observed in old caspase-2 knockout mice is due to aging because it occurred in an aging-dependent way, that is, no significant difference was observed at middle age among caspase-2 knockout and wild-type mice.
Harrison et al (1988) made an extensive review and examination of the biological traits that can potentially be used as biological markers of aging and found that hair re-growth ability was one of the most informative markers. The reduced hair re-growth ability in old caspase-2 knockout mice can thus be considered as a strong indication of the acceleration of aging.
A number of researchers have used subcutaneous fat content as a reflective marker of aging (Tyner et al., 2002; Trifunovic et al., 2004). In our opinion, this marker is inferior to maximum lifespan, bone mass and hair re-growth. Although people at their 80 or 90's do have significant loss of total body fat, many people in their 60's are obese. In addition, there is no reliable way to accurately measure the content of subcutaneous fat due to its uneven distribution. We measured the total body fat content of old mice at the time we measured BMD by DEXA scan. While the result is consistent with our other data, we consider it as only a piece of ancillary evidence supplemental to the study of lifespan, bone mass and hair re-growth ability.
Taken together, the results of lifespan, bone mass, hair re-growth and body fat study collaboratively suggest that caspase-2 deficiency affects the rate of aging in mice.
Since caspase-2 mediates oxidative stress-induced apoptosis (Lopez-Cruzan et al., 2005; Prasad et al., 2006), loss of caspase-2 function will compromise the animal's ability to remove oxidatively damaged cells through apoptosis. This may explain the increased level of oxidized proteins observed in caspase-2 knockout mice. What is particular noteworthy is that deletion of caspase-2 resulted in an increased number of osteoclasts. While osteoclasts are essential for the maintenance of a healthy skeletal system throughout an animal's life, excessive activity of osteoclasts accelerates the development of an aging-related phenotype (bone loss). On a broader spectrum, caspase-2 deficiency may affect aging or aging-related traits through a similar influence on other cells.
Caspase-2 is an initiator caspase that functions in the upstream of the apoptosis signaling cascade. Caspase-2 induces the release of mitochondrial proapoptotic proteins to cytosol, where these proteins activate the final execution phase of apoptosis (Baliga et al., 2004; Gao et al., 2005; Bonzon et al., 2006). Expression of caspase-2 gene has been detected in a broad range of tissues including brain, heart, kidney, lung and spleen (Bergeron et al., 1998). An apoptotic role of caspase-2 has been found in neurons, hepatocytes, fibroblasts, macrophages, lymphocytes, and oocytes (Troy et al., 2001; Guicciardi et al., 2005; Tu et al., 2006; Bergeron et al., 1998; Jesenberger et al., 2000). It is puzzling that the deletion of caspase-2 gene did not cause severe abnormality or prenatal death of the mice as seen in the knockout of other initiator caspases such as caspase-8 or -9 (Varfolomeev et al., 1998; Kuida et al., 1998). The explanation is that the function of caspase-2 can be compensated by other proteins/caspases in the caspase-2 null mice (Troy et al., 2001). However, such compensation, if exists, is not complete, because caspase-2 knockout mice did display significant changes at a later stage of life as revealed by this study.
How does caspase-2 regulate aging-related traits? As caspase-2 is an initiator caspase, loss of caspase-2 gene may hamper the activation of the executioner caspases, thus, compromise apoptosis. We have previously reported that the activities of both caspase-2 and caspase-3 in rat livers increase with age (Zhang et al., 2002b). Recently, we have found that loss of caspase-2 gene significantly hampered the activation of caspase-3 in the liver of aging mice (unpublished data). Therefore, caspase-2 may regulate aging-related traits by affecting the executioner caspase-3 and cell death.
Aging is a result of imbalanced physiological functions. Not only may the decrease of regenerative functions, but also the increase of degenerative functions contribute to the aging phenotype. In this regard, the bone represents an interesting example. Throughout an animal's life, the bone mass is maintained dynamically by the resorption of existing bones and the formation of new bones (Blair et al., 1993). With the progress of aging, the activity of bone resorption surpasses the activity of bone formation, which results in aged bone phenotypes such as osteoporosis and kyphosis. Recently, we have found that the inhibition of caspase-2 activity in primary osteoclasts significantly increased the viability of these cells (unpublished data). Since an increase in the number of osteoclasts will enhance bone resorption in aging animals, caspase-2 deficiency may accelerate the development of aging bone phenotypes by extending the survival of osteoclasts. On a broader spectrum, caspase-2 deficiency may accelerate the development of other aging-related traits with similar mechanisms. Future study will be directed to the identification of specific types of cells or tissues that underlie these observed phenotypes.
To conclude, we found that deletion of caspase-2, an important component of the intrinsic pathway of apoptosis, enhanced a number of aging-related traits commonly seen in premature aging animals. This study thus demonstrates for the first time that apoptosis plays an important role in aging.
Acknowledgements
We thank Drs. Junying Yuan and Carol Troy for providing caspase-2 knockout mice. We thank Kinton Armmer, Catherine Haskins, Vivian Diaz, Anuradha Soundararajan and Beryl M Story for technical help. This research is supported by a grant from National Institute on Aging (P01AG019316, B.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams CS, Horton WE., Jr. Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat. Rec. 1998;250:418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Baliga BC, Read SH, Kumar S. The biochemical mechanism of caspase-2 activation. Cell Death Differ. 2004;11:1234–1241. doi: 10.1038/sj.cdd.4401492. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL, Yuan J. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair HC, Schlesinger PH, Ross FP, Teitelbaum SL. Recent advances toward understanding osteoclast physiology. Clin. Orthop. Relat. Res. 1993;294:7–22. [PubMed] [Google Scholar]
- 5.Bonzon C, Bouchier-Hayes L, Pagliari LJ, Green DR, Newmeyer DD. Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death. Mol. Biol. Cell. 2006;17:2150–2157. doi: 10.1091/mbc.E05-12-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffed borders and resorb bone in mice. J. Clin. Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Carnevale V, Dicembrino F, Frusciante V, Chiodini I, Minisola S, Scillitani A. Different patterns of global and regional skeletal uptake of 99mTc-methylene diphosphonate with age: relevance to the pathogenesis of bone loss. J. Nucl. Med. 2000;41:1478–1483. [PubMed] [Google Scholar]
- 9.Chaudhuri AR, Khan IA, Luduena RF. Detection of disulfide bonds in bovine brain tubulin and their role in protein folding and microtubule assembly in vitro: a novel disulfide detection approach. Biochemistry. 2001;40:8834–8841. doi: 10.1021/bi0101603. [DOI] [PubMed] [Google Scholar]
- 10.Delmas PD, Schlemmer A, Gineyts E, Riis B, Christiansen C. Urinary excretion of pyridinoline crosslinks correlates with bone turnover measured on iliac crest biopsy in patients with vertebral osteoporosis. J. Bone Miner. Res. 1991;6:639–644. doi: 10.1002/jbmr.5650060615. [DOI] [PubMed] [Google Scholar]
- 11.Dlamini Z, Mbita Z, Ledwaba T. Can targeting apoptosis resolve the cancer saga? Fut. Oncol. 2005;1:339–349. doi: 10.1517/14796694.1.3.339. [DOI] [PubMed] [Google Scholar]
- 12.Fujino Y, Ozaki K, Yamamasu S, Ito F, Matsuoka I, Hayashi E, Nakamura H, Ogita S, Sato E, Inoue M. DNA fragmentation of oocytes in aged mice. Hum. Reprod. 1996;11:1480–1483. doi: 10.1093/oxfordjournals.humrep.a019421. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z, Shao Y, Jiang X. Essential roles of the Bcl-2 family of proteins in caspase-2-induced apoptosis. J. Biol. Chem. 2005;280:38271–38275. doi: 10.1074/jbc.M506488200. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. Embo J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrilov LA, Gavrilova NS. The Biology of Life Span: A Quantitative Approach. Harwood Academic Publisher; New York: 1991. [Google Scholar]
- 16.Guicciardi ME, Bronk SF, Werneburg NW, Yin XM, Gores GJ. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129:269–284. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Harrison DE, Archer JR. Biomarkers of aging: tissue markers. Future research needs, strategies, directions and priorities. Exp. Gerontol. 1988;23:309–325. doi: 10.1016/0531-5565(88)90034-4. [DOI] [PubMed] [Google Scholar]
- 18.Higami Y, Shimokawa I, Tomita M, Okimoto T, Koji T, Kobayashi N, Ikeda T. Aging accelerates but life-long dietary restriction suppresses apoptosis-related Fas expression on hepatocytes. Am. J. Pathol. 1997;151:659–663. [PMC free article] [PubMed] [Google Scholar]
- 19.Jesenberger V, Procyk KJ, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 2000;192:1035–1046. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Cruzan M, Zhang JH, Medina S, Ramanujan VK, Centonze VF, Masuda A, Herman B. Real Observation of Bio-Molecular Interaction. In: Imanish Y, editor. Proceedings of the International Symposium on Molecular Nanotechnology; Nara, Japan. November 2005.2005. pp. 217–232. [Google Scholar]
- 22.Muskhelishvili L, Hart RW, Turturro A, James SJ. Age-related changes in the intrinsic rate of apoptosis in livers of diet-restricted and ad libitumfed B6C3F1 mice. Am. J. Pathol. 1995;147:20–24. [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad V, Chandele A, Jagtap JC, Kumar PS, Shastry P. ROS-triggered caspase 2 activation and feedback amplification loop in beta-carotene-induced apoptosis. Free Radic. Biol. Med. 2006;41:431–442. doi: 10.1016/j.freeradbiomed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Rico H, Relea P, Crespo R, Revilla M, Villa LF, Arribas I, Usabiaga J. Biochemical markers of nutrition in type-I and type-II osteoporosis. J. Bone Joint Surg. Br. 1995;77:148–151. [PubMed] [Google Scholar]
- 25.Subramanian G, McAfee JG, Blair RJ, Kallfelz FA, Thomas FD. Technetium-99m-methylene diphosphonate--a superior agent for skeletal imaging: comparison with other technetium complexes. J. Nucl. Med. 1975;16:744–755. [PubMed] [Google Scholar]
- 26.Taglialatela G, Gegg M, Perez-Polo JR, Williams LR, Rose GM. Evidence for DNA fragmentation in the CNS of aged Fischer-344 rats. Neuroreport. 1996;7:977–980. doi: 10.1097/00001756-199604100-00004. [DOI] [PubMed] [Google Scholar]
- 27.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 28.Troy CM, Rabacchi SA, Hohl JB, Angelastro JM, Greene LA, Shelanski ML. Death in the balance: alternative participation of the caspase-2 and -9 pathways in neuronal death induced by nerve growth factor deprivation. J. Neurosci. 2001;21:5007–5016. doi: 10.1523/JNEUROSCI.21-14-05007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troy CM, Shelanski ML. Caspase-2 redux. Cell Death Differ. 2003;10:101–107. doi: 10.1038/sj.cdd.4401175. [DOI] [PubMed] [Google Scholar]
- 30.Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat. Cell Biol. 2006;8:72–77. doi: 10.1038/ncb1340. [DOI] [PubMed] [Google Scholar]
- 31.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 32.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham KB, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 33.Yildirim M, Gursoy R, Varoglu E, Oztasyonar Y, Cogalgil S. 99mTc-MDP bone SPECT in evaluation of the knee in asymptomatic soccer players. Br. J. Sports Med. 2004;38:15–18. doi: 10.1136/bjsm.2002.000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Herman B. Ageing and apoptosis. Mech. Ageing Dev. 2002a;123:245–260. doi: 10.1016/s0047-6374(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Chong E, Herman B. Age-associated increases in the activity of multiple caspases in Fisher 344 rat organs. Exp. Gerontol. 2002b;37:777–789. doi: 10.1016/s0531-5565(02)00013-x. [DOI] [PubMed] [Google Scholar]