Abstract
Background and Purpose
Hyperglycemia is linked to a worse outcome after ischemic stroke. Among the manifestations of brain damage caused by ischemia are blood-brain barrier (BBB) disruption and edema formation. Oxidative stress and matrix metalloproteinase-9 (MMP-9) activation are implicated in BBB dysfunction after ischemia/reperfusion injury. Our present study was designed to clarify the relationship among hyperglycemia, oxidative stress, and MMP-9 activation associated with BBB dysfunction after transient focal cerebral ischemia (tFCI).
Methods
We used a model of 60 minutes of middle cerebral artery occlusion on the following animals: normoglycemic wild-type rats, wild-type rats with hyperglycemia induced by streptozotocin, and human copper/zinc-superoxide dismutase (SOD1) transgenic rats with streptozotocin-induced hyperglycemia. We evaluated edema volume, Evans blue leakage, and oxidative stress, such as the carbonyl groups and oxidized hydroethidine (HEt), SOD activity, and gelatinolytic activity, including MMP-9.
Results
Hyperglycemia significantly increased edema volume and Evans blue leakage. Moreover, it enhanced the levels of the carbonyl groups, the oxidized HEt signals, and MMP-9 activity after tFCI without alteration in SOD activity. Gelatinolytic activity and oxidized HEt signals had a clear spatial relationship in the hyperglycemic rats. SOD1 overexpression reduced the hyperglycemia-enhanced Evans blue leakage and MMP-9 activation after tFCI.
Conclusions
Hyperglycemia increases oxidative stress and MMP-9 activity, exacerbating BBB dysfunction after ischemia/reperfusion injury. Superoxide overproduction may be a causal link among hyperglycemia, MMP-9 activation, and BBB dysfunction.
Keywords: hyperglycemia, oxidative stress, metalloproteinase, blood-brain barrier, cerebral ischemia
Introduction
Hyperglycemia at the time of ischemic stroke is linked to increased risk of mortality and poor functional recovery.1,2 Experimental studies have shown that preischemic hyperglycemia aggravates the development of damage after reperfusion.3,4 One of the manifestations of central nervous system damage caused by cerebral ischemia is the formation of brain edema, which is a result of the breakdown of the blood-brain barrier (BBB). Hyperglycemia has a deleterious effect on the BBB and causes edema formation after global ischemia.3,5
Oxidative stress is involved in the regulation of junctional proteins of endothelial cells.6,7 Reactive oxygen species (ROS), especially superoxide anions, are critical factors in BBB disruption and brain edema formation after transient focal cerebral ischemia (tFCI).8,9 In contrast, matrix metalloproteinases (MMPs), including MMP-2 (gelatinase A) and MMP-9 (gelatinase B), can degrade components of the extracellular matrix around the blood vessels.10 MMP-9 particularly, plays a pivotal role in the proteolytic degradation of the BBB after tFCI, as demonstrated by an experiment using MMP-9 null mice.11 Moreover, oxidative stress activates MMP-9 to mediate the breakdown of the BBB after tFCI.10,12
Recent reports showed that hyperglycemia enhances ROS that are associated with exacerbated neuronal damage after ischemia/reperfusion injury.13,14 However, the exact mechanisms between hyperglycemia and BBB disruption after tFCI are still unknown. In this study, we examined the hypothesis that hyperglycemia may enhance oxidative stress and MMP-9 activation that are associated with increased BBB permeability after tFCI.
Materials and Methods
Focal Cerebral Ischemia
The experimental protocol and procedures were approved by the Administrative Panel on Laboratory Animal Care of Stanford University. Adult male Sprague-Dawley rats (250 to 280 g) were subjected to tFCI by intraluminal middle cerebral artery (MCA) blockade with a 22.0-mm 3-0 surgical monofilament nylon suture.15 The animals were anesthetized with a nitrous oxide/oxygen/isoflurane mixture (69%/30%/2%) during surgical preparation. After 60 minutes of MCA occlusion (MCAO), cerebral blood flow was restored by removal of the nylon thread. The animals were divided into three groups: normoglycemic wild-type (Wt) rats; hyperglycemic Wt rats; hyperglycemic human copper/zinc (Cu/Zn) -superoxide dismutase (SOD) transgenic (Tg) rats (SOD1).
SOD1 Tg Rats
Heterozygous SOD1 Tg rats with a Sprague-Dawley background, carrying human SOD1 genes with a four- to six-fold increase in Cu/Zn-SOD, were derived from the founder stock described previously.16 There were no observable phenotypic differences, including in the cerebral vasculature, between the Tg rats and Wt littermates, as reported previously.16
Induction of Acute Hyperglycemia
Hyperglycemia was induced by intraperitoneal injection of streptozotocin (60 mg/kg; Sigma-Aldrich) 3 days before MCAO. The serum glucose level was measured with a One Touch Ultra Blood Glucose monitoring system (LifeScan, Inc.).
Examination of Neurological Symptoms
Neurological symptoms were examined in a blind manner 24 hours after reperfusion based on detection of hemiplegia and abnormal posture as follows; 0, normal; 1, forelimb flexion; 2, decreased resistance to lateral push and forelimb flexion; 3, same behavior as grade 2, with circling (n=7 each).17
Measurement of Infarct Volume and Edema Volume
Coronal sections were cut into 2-mm slices (n=7 each), which were immediately immersed in 2% 2,3,5-triphenyltetrazolium chloride at 37°C for 30 minutes. The infarct areas of each slice were separately summed and multiplied by the interval thickness to obtain infarct volumes.18 Edema volume was determined by subtracting the total volume of the non-ischemic hemisphere from that of the ischemic hemisphere.18
Determination of BBB Permeability With the Use of Evans Blue
Immediately after reperfusion, 4 mL/kg of 2% Evans blue (Sigma-Aldrich) in normal saline were injected into the right jugular vein (n=6 each). The animals were killed 24 hours after reperfusion. For quantitative measurements, the brain hemispheres (ischemic side) were homogenized in 3 mL of N,N-dimethyl-formamide (Sigma-Aldrich), incubated for 18 hours at 55°C, and centrifuged.19 The supernatants were analyzed at 620 nm using a spectrophotometer.
Western Blot Analysis
Whole-cell fractions were obtained from the entire MCA territory (ischemic side) (n=6 each).15 Equal amounts of the samples were loaded per lane. The primary antibodies were an anti-Cu/Zn-SOD antibody (1:8000; Nventa), an anti-manganese (Mn) SOD antibody (1:4000; Nventa), and an anti-β-actin antibody (1:10000; Sigma-Aldrich), or an anti-cyclooxygenase (COX) antibody (1: 2500; Molecular Probes, Inc.). Western blots were performed with horseradish peroxidase-conjugated immunoglobulin G (Amersham) with the use of enhanced chemiluminescence detection reagents (Amersham).
Detection of Oxidative Protein Damage to the Brain
Samples were prepared as described in the Western blot analysi1s (n=6). With the use of a commercial kit (Chemicon International), we observed the carbonyl groups as indicators of oxidative protein damage. The samples were incubated with 2,4-dinitrophenylhydrazone (DNP) and the DNP-derivatized carbonyl groups were specifically detected by Western blot using an anti-DNP antibody. We analyzed the density of each lane by the total expression of DNP.
SOD Activity Assay
Samples were prepared as described in the Western blot analysis (n=6 each). Total SOD activity was measured using a commercial kit (Calbiochem) with a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Gel Zymography
Extraction of MMP from the brains was performed as described previously (n=6 each).19 Supernatants obtained from homogenized brain tissues were incubated with gelatin-Sepharose 4B (GE Healthcare). After incubation and centrifugation, the samples were resuspended in elution buffer. The same protein quantity from each sample was loaded and separated on 10% Tris-glycine gels with 0.1% gelatin (Invitrogen). A mixture of MMP-9 and MMP-2 (CC073; Chemicon International) was used as a gelatinase standard. Images of the gels were scanned using a densitometer (GS-700; Bio-Rad Laboratories) and quantification was performed using Multi-Analyst 1.0 software (Bio-Rad Laboratories).
In Situ Zymography
In situ gelatinase activity was detected on frozen brain sections 20 μm thick with the use of a commercial kit (Molecular Probes) (n=5 each). The sections were incubated with DQ gelatin (Invitrogen) fluorescein conjugate at 37°C for 2 hours.12 Cleavage of DQ gelatin caused by MMPs resulted in a green fluorescence, observed at excitation of 495 nm and emission of > 515 nm.
In Situ Detection of Superoxide Anion Production and Double Labeling with Gelatinase Activity
The brain sections were prepared as those for in situ zymography (n=5). Superoxide anion production in the brain was measured as oxidized hydroethidine (HEt).20 The sections were immediately incubated with HEt (10 μmol/L; Sigma-Aldrich) in phosphate-buffered saline for 30 minutes at 37°C. After incubation, the sections were incubated with DQ gelatin fluorescein conjugate. Oxidized HEt fluorescence was observed at excitation of 510 nm and emission of > 580 nm and quantified using NIH image software (version 1.62; National Institutes of Health).21
Quantification and Statistical Analysis
The data are expressed as mean ± SD. Comparison between two groups was achieved using Student’s t test. Significance was accepted with P<0.05. Comparison between normoglycemic and hyperglycemic rats was achieved using analysis of variance for repeated measure with Fisher’s protected least-significant difference post hoc analysis (StatView). Significance was accepted with P<0.05.
Results
Physiological Parameters
No significant difference was seen in physiological parameters, such as mean arterial blood pressure, rectal temperature, pH, PO2, and PCO2, among the normoglycemic Wt rats, the hyperglycemic Wt rats, and the hyperglycemic SOD1 Tg rats before, during, or after MCAO (Table). There was also no significant difference in regional cerebral blood flow among the groups (data not shown). Serum glucose levels were significantly higher in the hyperglycemic Wt rats and in the hyperglycemic SOD1 Tg rats at each time point compared with the normoglycemic Wt rats (P<0.05; Figure 1A). No difference in the level of serum glucose was observed between the hyperglycemic Wt rats and the hyperglycemic SOD1 Tg rats at any time point (Figure 1A).
TABLE.
Physiological Data
| Time points | Parameters | NG (n=6) | HG (n=6) | HGT (n=6) |
|---|---|---|---|---|
| 10 minutes before MCAO | ||||
| MABP (mm Hg) | 92.5 ± 5.5 | 91.5 ± 6.8 | 93.0 ± 4.7 | |
| T (° C) | 37.1 ± 0.2 | 37.1 ± 0.4 | 37.1 ± 0.4 | |
| pH | 7.37 ± 0.03 | 7.38 ± 0.03 | 7.37 ± 0.06 | |
| PO2 (mm Hg) | 113.1 ± 13.2 | 116.7 ± 8.1 | 111.9 ± 13.8 | |
| PCO2 (mm Hg) | 42.1 ± 2.9 | 42.2 ± 1.7 | 42.7 ± 1.2 | |
| 30 minutes after MCAO | ||||
| MABP (mm Hg) | 83.7 ± 3.1 | 86.7 ± 8.5 | 88.0 ± 4.1 | |
| T (° C) | 37.2 ± 0.3 | 37.4 ± 0.2 | 37.2 ± 0.4 | |
| pH | 7.35 ± 0.03 | 7.36 ± 0.04 | 7.35 ± 0.02 | |
| PO2 (mm Hg) | 105.0 ± 7.1 | 97.2 ± 6.1 | 101.6 ± 8.2 | |
| PCO2 (mm Hg) | 43.8 ± 3.8 | 44.4 ± 3.6 | 44.8 ± 3.0 | |
| 10 minutes after reperfusion | ||||
| MABP (mm Hg) | 89.5 ± 10.3 | 86.7 ± 12.5 | 90.0 ± 6.0 | |
| T (° C) | 37.2 ± 0.3 | 37.0 ± 0.2 | 37.1 ± 0.4 | |
| pH | 7.36 ± 0.03 | 7.36 ± 0.03 | 7.36 ± 0.02 | |
| PO2 (mm Hg) | 99.7 ± 14.9 | 93.5 ± 8.3 | 95.4 ± 6.9 | |
| PCO2 (mm Hg) | 43.8 ± 5.5 | 45.3 ± 2.1 | 45.2 ± 3.0 |
MABP indicates mean arterial blood pressure; T, rectal temperature; NG, normoglycemic Wt rats; HG, hyperglycemic Wt rats; HGT, hyperglycemic SOD1 Tg rats.
Figure 1.
A, Serum glucose level in normoglycemic Wt rats (NG), hyperglycemic Wt rats (HG), and hyperglycemic SOD1 Tg rats (HGT) before, during, or after MCAO (*P<0.05). B, Neurological scores 24 hours after tFCI in NG and HG rats. Each symbol depicts the individual score of a single animal (*P<0.05). C, Representative photographs of 2,3,5-triphenyltetrazolium chloride staining 24 hours after tFCI in NG and HG rats. Bar = 1 cm. D, Infarct volume 24 hours after tFCI in NG and HG rats (*P<0.05). E, Edema volume 24 hours after tFCI in NG and HG rats (*P<0.05). F, Representative photographs of Evans blue extravasation in the brains and coronal sections (bregma +0.70 mm) of NG and HG rats 24 hours after tFCI. Bars = 5 mm. G, Quantitative assay of Evans blue leakage in NG and HG rats 24 hours after tFCI (*P<0.05).
Hyperglycemia Aggravated Neurological Scores, Infarction Volume, Edema Volume, and BBB Permeability After tFCI
Hyperglycemia worsened the neurological scores after tFCI (P<0.05; Figure 1B) and significantly increased infarct volume and edema volume 24 hours after tFCI (P<0.05; Figure 1C, 1D, and 1E). Moreover, hyperglycemia promoted Evans blue leakage after reperfusion (P<0.05; Figure 1F and 1G).
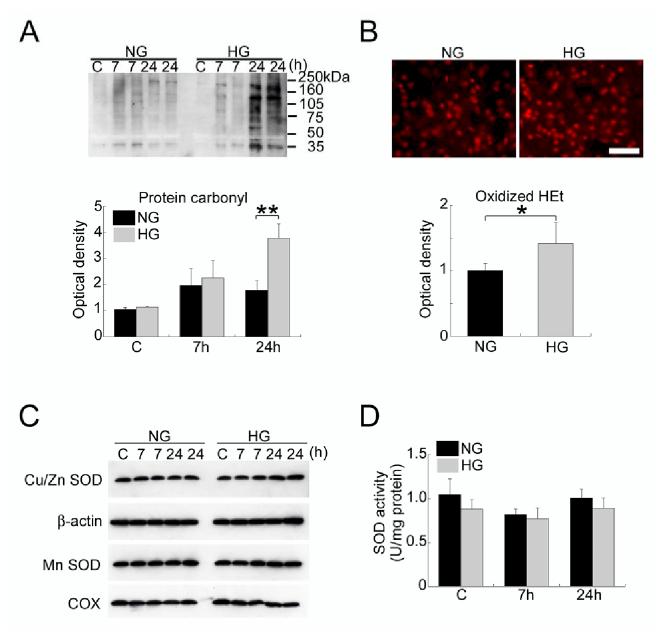
Hyperglycemia Enhanced Oxidative Stress Without Alteration in SOD Activity After tFCI
Hyperglycemia did not change the levels of the carbonyl groups in the sham control brains or the brains subjected to 7 hours of reperfusion, but did significantly increase the levels 24 hours after tFCI (P<0.01; Figure 2A). In addition, hyperglycemia remarkably increased the oxidized HEt signals in the ischemic area at 24 hours (P<0.05; Figure 2B). In contrast, hyperglycemia did not affect the levels of Cu/Zn-SOD, MnSOD immunoreactivity, or SOD activity in the sham control brains or the brains subjected to 7 or 24 hours of reperfusion after tFCI (Figure 2C and 2D).
Figure 2.
A, Western blot analysis of the carbonyl groups in normoglycemic Wt rats (NG) and hyperglycemic Wt rats (HG). Hyperglycemia markedly raised the levels of the carbonyl groups 24 hours after tFCI (**P<0.01). C indicates control. B, In situ detection of oxidized HEt in the ischemic area of NG and HG rats 24 hours after tFCI. The oxidized HEt signals were significantly increased in the HG rats compared with the NG rats (*P<0.05). Bar = 50 μm. C, Western blot analysis of Cu/Zn-SOD and MnSOD in NG and HG rats. There was no significant difference in Cu/Zn-SOD or MnSOD immunoreactivity between the NG and HG rats. β-actin and COX were used as internal controls. D, Assay of total SOD activity in NG and HG rats. Hyperglycemia had no significant effect on SOD activity in the rat brains. Note: Duplicate samples were run at 7 and 24 hours (A and C).
Hyperglycemia Promoted MMP-9 Activity After tFCI
Zymography showed two MMP-9 bands (92 kDa and 88 kDa). Total MMP-9 levels (i.e., both bands together) were quantified. Hyperglycemia did not change MMP-9 activity in the sham control brains or the brains subjected to 7 hours of reperfusion after tFCI, but did remarkably enhance MMP-9 activity 24 hours after tFCI (P<0.01; Figure 3A). No difference in MMP-2 activity (68 kDa) was observed between the normoglycemic Wt rats and the hyperglycemic Wt rats (Figure 3A). In situ zymography demonstrated that hyperglycemia did not change the level of gelatinase activity in the sham control brains, but increased the activity in vessels and cells of the ischemic area 24 hours after tFCI (Figure 3B). Almost all of the signals of gelatinase activity in vessels and cells were colocalized with the oxidized HEt signals 24 hours after tFCI in the hyperglycemic Wt rats (Figure 3C).
Figure 3.

A, Zymographic analysis in normoglycemic Wt rats (NG) and hyperglycemic Wt rats (HG). Hyperglycemia significantly increased MMP-9 activity 24 hours after tFCI (**P<0.01), but did not change MMP-2 activity in any group. MMP-9 bands (92 kDa and 88 kDa) and MMP-2 bands (68 kDa) were detected. C indicates control; hSt, human gelatinase standard. B, Representative photomicrographs of gelatinase activity in the sham controls and the ischemic areas of NG and HG rats 24 hours after tFCI. In the sham control brains, there was no difference in gelatinase activity between the NG and HG rats. After 24 hours of reperfusion, gelatinase activity in vessels (arrows) and cells (arrowheads) was prominent in the HG rats compared with the NG rats. Bar = 100 μm. C, Colocalization of gelatinase activity and oxidized HEt in vessels (arrows) and cells (arrowheads) of the HG rats 24 hours after tFCI. Bar = 100 μm.
Overexpression of SOD1 in Hyperglycemic Rats Reduced BBB Permeability and MMP-9 Activation after tFCI
The level of SOD activity was prominent in the hyperglycemic SOD1 Tg rats compared with the hyperglycemic Wt rats (P<0.01; Figure 4A). MMP-9 activity and Evans blue leakage were significantly reduced in the hyperglycemic SOD1 Tg rats compared with the hyperglycemic Wt rats 24 hours after tFCI (P<0.05) (Figure 4B and 4C).
Figure 4.

A, Assay of SOD activity in hyperglycemic Wt rats (HG) and hyperglycemic SOD1 Tg rats (HGT) (**P<0.01). B, Zymographic analysis in HG and HGT rats 24 hours after tFCI. MMP-9 activity was significantly reduced in the HGT rats compared with the HG rats (*P<0.05). C, Quantitative analysis of Evans blue leakage in the HG and HGT rats 24 hours after tFCI. The level of Evans blue leakage was remarkably decreased in the HGT rats compared with the HG rats (*P<0.05).
Discussion
The present study demonstrated that hyperglycemia significantly aggravated BBB permeability, edema formation, and neurological symptoms after tFCI. We believe that this model is ideal for investigating the mechanisms of hyperglycemia-enhanced BBB breakdown after ischemia/reperfusion injury. When BBB integrity is lost, inflammatory cells and fluid penetrate the brain, causing edema and cell death.22 Moreover, brain edema is known to be an important factor in the acute phase of mortality because of the development of severe brain swelling and herniation. Therefore, these findings suggest that hyperglycemia worsens outcome by increasing BBB permeability during reperfusion.
Hyperglycemia is thought to enhance the generation of ROS such as hydroxyl radicals and superoxide during reperfusion.13,14 In this study, hyperglycemia significantly raised the level of the carbonyl groups, i.e., oxidized proteins, after tFCI. During reperfusion, production of ROS and the activity of antioxidants are implicated in oxidative stress-induced injury.23 The present study demonstrated that hyperglycemia increased oxidized HEt signals after tFCI, suggesting that hyperglycemia enhances superoxide production during reperfusion. Pro-oxidant enzymes, such as COX-2 and nicotinamide-adenine dinucleotide phosphate oxidase, generate superoxide in the later stage of ischemic reperfusion.23 Hyperglycemia has been shown to increase the expression of COX-2 and nicotinamide-adenine dinucleotide phosphate oxidase.24,25 Moreover, hyperglycemia is believed to be related to higher lactic acid accumulation, leading to mitochondrial dysfunction.26 Mitochondria may also be the sites of hyperglycemia-enhanced superoxide production. Although we cannot rule out the possibility that hyperglycemia exerts deleterious effects on other antioxidants, we demonstrated that hyperglycemia caused no significant change in SOD activity after tFCI. This finding is consistent with the view that SOD, Cu/Zn-SOD in particular, is highly resistant to acidic pH.
In this study, the activity of MMP-9 (but not MMP-2) in the ischemic area was preferentially enhanced by hyperglycemia during reperfusion. Increased MMP-9 activity reduces a junctional protein in endothelial cells, which results in BBB disruption after tFCI.11 This information, taken together, suggests that hyperglycemia may aggravate BBB disruption partly through increased MMP-9 activity after ischemia/reperfusion injury. ROS, especially superoxide anions, are implicated in MMP-9 activation, BBB disruption, and edema formation after cerebral ischemia, as demonstrated by the experiments using SOD1-deficient or MnSOD (SOD2) -deficient mice.8,9,12 In the present study, we also showed that overexpression of SOD1 attenuated MMP-9 activation and BBB permeability, enhanced by hyperglycemia. Furthermore, a double fluorescence study showed the clear spatial relationship between gelatinase activation and superoxide production in vessels and cells. These findings suggest that superoxide overproduction is a causal link among preischemic hyperglycemia, MMP-9 activation, and BBB dysfunction after tFCI.
In conclusion, hyperglycemia increases oxidative stress and MMP-9 activity, aggravating BBB dysfunction, after ischemia/reperfusion injury. Antioxidant agents may help to prevent hyperglycemia-enhanced BBB dysfunction, edema formation, and a worse outcome in patients with ischemic stroke.
Acknowledgments
This work was supported by National Institutes of Health grants P50NS14543, RO1 NS25372, RO1 NS36147, and RO1 NS38653. We thank Liza Reola and Bernard Calagui for technical assistance, Cheryl Christensen for editorial assistance, and Elizabeth Hoyte for figure preparation.
Footnotes
Disclaimer:
This is an un-copyedited author manuscript that was accepted for publication in Stroke, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Stroke. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD, for the Trial of ORG in Acute Stroke Treatment (TOAST) Investigators Acute blood glucose level and outcome from ischemic stroke. Neurology. 1999;52:280–284. doi: 10.1212/wnl.52.2.280. [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients. A systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 3.Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: a neuropathologic study in the rat. Neurology. 1982;32:1239–1246. doi: 10.1212/wnl.32.11.1239. [DOI] [PubMed] [Google Scholar]
- 4.Yip PK, He YY, Hsu CY, Garg N, Marangos P, Hogan EL. Effect of plasma glucose on infarct size in focal cerebral ischemia-reperfusion. Neurology. 1991;41:899–905. doi: 10.1212/wnl.41.6.899. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich WD, Alonso O, Busto R. Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke. 1993;24:111–116. doi: 10.1161/01.str.24.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:H2820–H2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- 7.Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmári E, Traweger A, Wejksza K, Bauer H-C. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol. 2005;25:129–139. doi: 10.1007/s10571-004-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo T, Reaume AG, Huang T-T, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier CM, Hsieh L, Crandall T, Narasimhan P, Chan PH. Evaluating therapeutic targets for reperfusion-related brain hemorrhage. Ann Neurol. 2006;59:929–938. doi: 10.1002/ana.20850. [DOI] [PubMed] [Google Scholar]
- 10.Liu KJ, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39:71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Asahi M, Wang X, Mori T, Sumii T, Jung J-C, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasche Y, Copin J-C, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Wei J, Huang NC, Quast MJ. Hydroxyl radical formation in hyperglycemic rats during middle cerebral artery occlusion/reperfusion. Free Radic Biol Med. 1997;23:986–995. doi: 10.1016/s0891-5849(97)00127-5. [DOI] [PubMed] [Google Scholar]
- 14.Muranyi M, Li P-A. Hyperglycemia increases superoxide production in the CA1 pyramidal neurons after global cerebral ischemia. Neurosci Lett. 2006;393:119–121. doi: 10.1016/j.neulet.2005.09.079. [DOI] [PubMed] [Google Scholar]
- 15.Kamada H, Nito C, Endo H, Chan PH. Bad as a converging signaling molecule between survival PI3-K/Akt and death JNK in neurons after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600367. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 18.Lin T-N, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 19.Kim GW, Gasche Y, Grzeschik S, Copin J-C, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J Neurosci. 2003;23:8733–8742. doi: 10.1523/JNEUROSCI.23-25-08733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulet R, Gentile MT, Vecchione C, Distaso M, Aretini A, Fratta L, Russo G, Echart C, Maffei A, De Simoni MG, Lembo G. Acute hypertension induces oxidative stress in brain tissues. J Cereb Blood Flow Metab. 2006;26:253–262. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara T, Noshita N, Lewén A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishman RA. Brain edema. N Engl J Med. 1975;293:706–711. doi: 10.1056/NEJM197510022931407. [DOI] [PubMed] [Google Scholar]
- 23.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R-L, Lu C-Z, Ren H-M, Xiao B-G. Metabolic changes of arachidonic acid after cerebral ischemia-reperfusion in diabetic rats. Exp Neurol. 2003;184:746–752. doi: 10.1016/S0014-4886(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 25.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RE, Tan WK, Martin HS, Meyer FB. Effects of glucose and Pao2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke. 1999;30:160–170. doi: 10.1161/01.str.30.1.160. [DOI] [PubMed] [Google Scholar]