Abstract
Some neurons in the visual cortex alter their spiking rate according to the perceptual interpretation of an observed stimulus, rather than its physical structure alone. Experiments in monkeys have suggested that, although the proportion of neurons showing this effect differs greatly between cortical areas, this proportion remains similar across different stimuli. These findings have raised the intriguing questions of whether the same neurons always participate in the disambiguation of sensory patterns and whether such neurons might represent a special class of cortical cells that relay perceptual signals to higher cortical areas. Here we explore this question by measuring activity in the middle temporal cortex of monkeys and asking to what degree the percept-related responses of individual neurons depend upon the specific sensory input. In contrast to our expectations, we found that even small differences in the stimuli led to significant changes in the signaling of the perceptual state by single neurons. We conclude that nearly all feature-responsive neurons in this area, rather than a select subset, can contribute to the resolution of sensory conflict, and that the role of individual cells in signaling the perceptual outcome is tightly linked to the fine details of the stimuli involved.
Keywords: binocular rivalry, neurophysiology, perceptual organization, visual perception
What aspects of neural activity in the brain are most directly responsible for producing and shaping the contents of our perception? This question is central to our understanding of how to link basic sensory processing with our subjective experience (1), and clear answers have thus far proven elusive. Visually bistable patterns, in which ambiguous or inherently conflicting (rivalrous) physical structure give rise to an ever-changing percept, have played an important role in exploring the neural roots of visual perception (2–4). For example, numerous electrophysiological experiments have used such patterns to dissociate constant sensory stimulation from a time-varying percept, often relying upon trained macaque monkeys to report how the stimulus appears at each moment. These experiments have revealed that the activity of a small proportion of neurons in the earliest cortical areas reflects a perceptually dominant stimulus, but that the number increases at higher processing stages (3, 5–13). Interestingly, it appears that the proportion of cells showing such perceptual modulation is roughly constant for a given area and does not depend greatly upon which bistable stimulus is used for testing. At least two different paradigms have been used in these types of studies. In the case of binocular rivalry, the two eyes view dissimilar patterns, and perceptual dominance alternates between each monocular view. In the case of bistable structure from motion, a half-transparent object rotating ambiguously in depth provokes alternations in the perceived direction of rotation. As it turns out, in the motion-sensitive medial temporal (MT) cortical area, 40%–50% of directionally tuned neurons showed modulation during binocular rivalry (10), with a similar proportion found by using 3D structure from motion (7, 9). In contrast, a considerably smaller fraction of modulating neurons was found in the primary visual cortex revealed by using both paradigms (6, 8, 12).
Why might the cortical expression of bistable perception be so similar for these rather different patterns? A parsimonious explanation that has been previously suggested (1) is that there exists a specialized perceptual network in the brain in which neurons have designated roles in perceptual signaling. Such a network might, for example, be associated with a particular neurochemistry or connectivity that is in place to effectively transmit disambiguated visual information to other brain structures. In this case, one might expect a neuron participating in such a network to consistently show perceptual modulation as long as the alternate perceptual states tap into its sensory tuning. A purely “sensory” neuron, in contrast, would respond only according to the stimulus present at any point in time and show no such modulation. Alternatively, it is possible that individual cells do not have fixed roles in perception at all, and thereby vary in their degree of perceptual modulation according to the specific stimulus conflict at hand.
Here we address this question by testing individual neurons with up to four different bistable patterns in the same session, asking whether a neuron showing perceptual modulation for one such stimulus would also have a high probability of showing similar modulation for another. We monitored neurons in the MT cortical area of monkeys presented with several binocular motion rivalry stimuli in the same session. Specifically, we used the robust paradigm of flash suppression (11, 14) to generate alternate perceptual states in which one or another direction of motion would be perceived during a period of binocular rivalry. In contrast to the previous predictions, we found that the perceptual modulation of a neuron with one rivalry pattern had very little bearing on the modulation with another, although the component stimuli were matched in terms of the neuron's basic sensory responses. This observation held true even when the percepts were identical or nearly identical, suggesting that the same perceptual state can arise from different underlying activity patterns within this area. These findings argue against the existence of a network in the visual cortex that is designated for expressing and relaying perceptual signals, and indicate instead that nearly all neurons in this area can carry perceptual signals depending upon the precise nature of the stimulus conflict.
Results
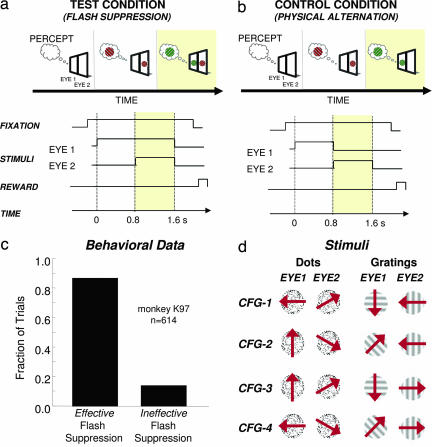
We monitored activity from the MT cortical area in two cerebral hemispheres of two macaque monkeys. One hundred seventy-two recording sites were recorded, from which 126 stimulus-responsive single units were isolated (88 for monkey K97 and 38 for monkey E00). Neurons were monitored ≤4 h, during which time the responses to several different binocular rivalry stimuli were tested. The stimuli consisted of either random dots or sinusoidal gratings moving in different directions in the two eyes (see Fig. 1). To ensure good control of the animals' perception on each trial, we used the paradigm of binocular rivalry flash suppression (BRFS), where two dissimilar monocular stimuli are presented with an asynchronous onset (Fig. 1a). When the delay time is sufficiently long, such as the 850 msec used in this study, perception in BRFS is reliably biased toward seeing the second stimulus in both humans (14) and monkeys (11).
Fig. 1.
Experimental paradigm. (a) (Upper) Monkeys were shown dichoptic grating patches or random dot fields by using a mirror stereoscope. BRFS test trials start with a monocular presentation of one of the two motion stimuli for several hundred milliseconds. After this period, the second stimulus is turned on in the other eye. The resulting percept is a stimulus sequence, during which one pattern is replaced by the other one. By reversing the temporal order between the stimuli, it is possible to raise each rival stimulus to awareness while keeping stimulation constant. (Lower) Time course of BRFS paradigm. (b) (Upper) Physical alternation control consists of a sequential monocular presentation of the two rival stimuli. The resulting perceptual sequence is similar to that evoked by BRFS with the same set of stimuli, but at no point is there more than one stimulus on the screen. (Lower) Time course of all experimentally relevant events for the physical alternation control condition. (c) Monkey psychophysics. The monkey was trained to report the physical presence of red or green stimuli and disregard stimuli that resemble interocular mixtures between the two. BRFS was instigated between various red and green stimuli, and successful trials (i.e., the monkey indicated a reversal from a red to a green stimulus and vice versa) were compared against unsuccessful ones (where no perceptual change was reported). Note that the fraction of reported trials with successful BRFS is ≈90%. (d) Stimulus set used for comparing perceptual modulation across four different stimulus configurations (CFG 1–4). The monocular patterns that were pitted against each other consisted of either circular patches of random dots that were moving coherently in one of four different directions (dots) or grating patches with the same outline drifting in four different directions (gratings).
Four different direction combinations (henceforth “configurations”) were presented in binocular conflict during pseudorandomized trials of flash suppression. We were thus able to investigate neural responses to alternate perceptual states of several rivalry stimuli and compare the activity differences to those expected based on the responses of the neuron to the corresponding component stimuli (Fig. 1b). Importantly, we could also determine whether individual cells would show similar levels of perceptual modulation for different rivalry configurations, as might be expected if they had a fixed role in the resolution of perceptual ambiguity. Due to the robustness of the BRFS technique, we did not require the animals to signal their percepts on each trial during testing, although we verified the consistent biasing of perception in one monkey (K97) during separate psychophysical testing (Fig. 1c). Figure 1 a and b shows the two conditions used in the experiment. In the “test” condition, neural responses were compared during the alternate perceptual states in binocular rivalry brought about by differential monocular adaptation (i.e., BRFS). In the “control” condition, there was no binocular rivalry, but simply a replacement of one monocular stimulus by another shown to the opposite eye. Rivalry stimuli consisted of either moving dots or grating patterns that differed by at least 45° in their direction of drift in the two eyes (see Fig. 1d). The control condition thus mimicked the sequential percept of the test condition, but there was no conflict present in need of perceptual resolution.
We first examined whether MT neurons responded differentially to the alternate percepts during flash suppression. In comparing the neural responses between the test and control conditions, we focused on the activity level following the onset of the second stimulus (indicated in yellow in Fig. 1 a and b), which marks the beginning of the rivalry period, and hence the alternate perceptual states. In the control condition, the cell's sensory preferences predicted activity differences during this period, and stimuli were categorized as effective (“preferred”) or ineffective (“null”) in activating the neuron based on the mean change in firing rate they elicited. In the test condition, where rivaling preferred and null stimuli are simultaneously present in opposite eyes, the expectations are less clear. Neurons showing no differentiated firing during alternate perceptual conditions might be relegated to pure sensory analysis, whereas those observed to alter their firing according to the percept might play a direct role in perception. It is important to note that, in conducting this test, we restricted our analysis to only those neurons that showed significant sensory tuning (i.e., significantly different mean responses) to the competing directions making up the binocular rivalry pairs.
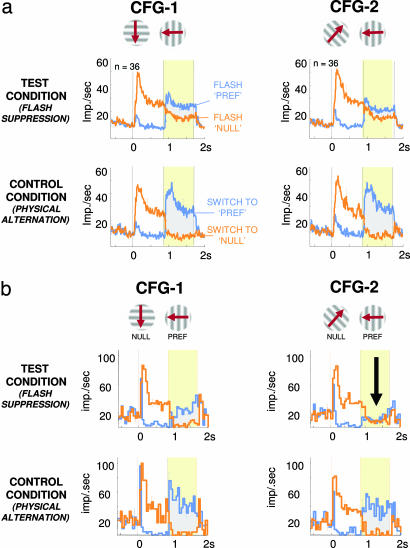
In accordance with previous results, the population activity in MT showed some differences between the two perceptual states. This difference was observed during flash suppression in both configurations shown in Fig. 2a and supporting information (SI) Fig. 11c (gray areas). Control experiments verified that the observed modulation was indeed related to the perceptual state, rather than basic sensory responses or neural spiking adaptation (see SI Fig. 10). Also in agreement with the previous studies, the magnitude of modulation corresponding to alternate perceptual states was small compared with the physical modulation in the control condition, despite the nearly identical visual appearance of the stimulus sequence in the two conditions.
Fig. 2.
Population responses and single-neuron example for the test and control conditions with two different stimulus configurations. (a) Population response of MT neurons to BRFS (both monkeys). (Middle and Bottom) Plots depict average spike density functions for 36 neurons, with significant selectivity for the two rivalry pairs (Top) (CFG-1 and CFG-2; see SI Fig. 11c for population responses to the random dot stimuli). (Middle) Average activity levels during BRFS. (Bottom) Mean activity during physical alternation (error bars depict SEM spaced in 500-msec increments). (Left Middle and Left Bottom) Responses to the stimulus configuration represented above (CFG-1). Same neurons' responses to flash suppression (Right Middle) and the corresponding control alternation for the alternative stimulus configuration (Right Bottom) (CFG-2). Note that in all cases the monkeys perceived a stimulus sequence, during which either the nonpreferred stimulus is replaced by the preferred stimulus (blue trace) or the preferred stimulus is followed by the nonpreferred pattern (orange trace). The period indicated in yellow corresponds to the time window of identical stimulation during BRFS, which includes the time period used for further analysis (see Methods). The difference in firing rate in response to both stimulus configurations correlates with the perceptual difference evoked by the two flash suppression conditions. (b) Single-unit example of stimulus-dependent perceptual modulation. Peristimulus time histograms depict mean firing rates for BRFS (Middle) and physical alternation (Bottom) of one example neuron (monkey K97). Action potentials were collected in bins of 50-msec size, which were averaged across trials. (Left Middle and Left Bottom) Responses to the stimulus configuration CFG-1 as depicted in Top. (Right Middle and Right Bottom) Mean responses to stimulus configuration CFG-2 (see SI Fig. 9b for example responses to the random dot stimuli). Note that the preferred stimulus did not change, whereas the nonpreferred stimulus was replaced by another stimulus that was not effective when presented in isolation. Nevertheless, perceptual modulation during perceptual dominance of the preferred pattern hinged on the nonpreferred pattern (as highlighted by the black arrow), although it has no direct effect on the activation of the cell (see Bottom).
Importantly, we next investigated whether a neuron showing perceptual modulation during flash suppression for one stimulus configuration would show similar modulation for another configuration consisting of different motion components. Specifically, we examined neurons for which the control stimulation revealed that they were tuned for multiple configurations (Fig. 2b Bottom, gray areas), and asked whether the degree of perceptual modulation would be similar for the two corresponding test configurations. Surprisingly, we found that neuronal responses were often completely different for different configurations in the test condition, although the control conditions were matched in their tuning. This effect can be seen in Fig. 2b, where the level of perceptual modulation for the two configurations was very different in the test condition (gray areas, Middle), despite the similar modulation in the two configurations during the control condition (gray areas, Bottom). In particular, this neuron showed strong modulation for the two different percepts in CFG-1, but none at all for CFG-2 (see SI Fig. 9 for more single-neuron examples).
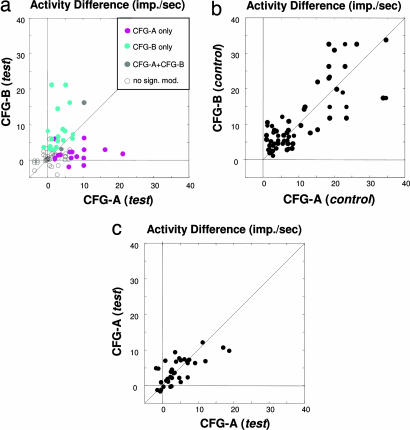
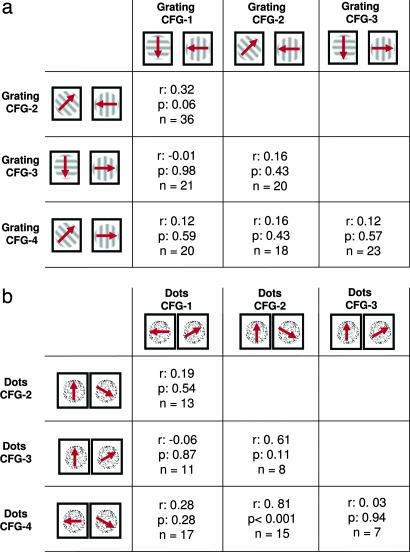
In fact, across the population of 117 neurons (85 with the grating stimuli and 32 with the random dots), the responses of a single neuron to different pairs of rivalry stimuli were almost completely uncorrelated, as can be seen in Fig. 3a (see also SI Fig. 6b). This scatter plot shows all combinations of stimulus configurations over the population of cells for which significant response differences were observed in the control condition (see Table 1 for the separate analysis of all individual rivalry pairs and SI Fig. 5 for an alternate method of analysis). Neurons showing perceptual modulation for one stimulus configuration often showed little if any such modulation for another. This result stands in contrast to the analogous plot made between different configurations in the control condition, shown in Fig. 3b. This figure shows that the very same neurons that show little correlation during flash suppression are highly correlated in their responses during physical alternation of the corresponding stimuli (see also SI Fig. 6 for similar results with a different set of rivalry stimuli). The correlation coefficients between all pairs of stimuli tested are shown in Table 1 for both the grating and random dot stimuli. Remarkably, we found that in only one case was there a significant correlation in the modulation observed between different stimulus configurations. In computing the population data, it is important to keep in mind that single-unit responses in the visual cortex are highly variable, and there is the danger that trial-to-trial variability could lead one to believe that responses to the two different stimulus configurations were significantly different, when, in fact, they were not. To ensure that spiking variability did not contribute to this observation, we applied the same analysis again, but for trials that all came from the same (test) condition from the same stimulus configuration. For each neuron, we randomly divided all of the trials in half and made a scatter plot of the means for the two halves. The results, shown in Fig. 3c, demonstrate the expected strong correlation across the population, proving that the results in Fig. 3a cannot be attributed to response variability (i.e., “noise”).
Fig. 3.
Most neurons do not transfer perceptual modulation between different BRFS configurations (data from monkey E00 by using the random dot motion stimuli; see SI Fig. 6 for data of both monkeys and the grating stimuli). (a) Scatter plot of mean activity difference (in spikes per sec) between the preferred and nonpreferred stimulus for the time period of 200–800 msec following flash suppression (resulting in identical stimulation with both stimuli on the screen). Each dot represents the average firing rates of one neuron. All significant response differences are color coded in gray (significant response differences for both stimulus configurations), cyan (significant response differences for CFG-B only), and magenta (significant responses for CFG-A only). The plot is a superposition of six plots obtained by individual stimulus comparisons. Accordingly, the CFG-A and CFG-B correspond to the following stimulus pair comparisons: configuration 3 vs. 4, 4 vs. 2, 3 vs. 2, 1 vs. 2, 3 vs. 1, and 1 vs. 4. Note that n (71) corresponds to the number of comparisons, rather than the absolute number of neurons (32). Neurons that were selective for more than two of the tested configurations are represented multiple times. There was no correlation between the response levels for each stimulus configuration (r = 0.2, P = 0.1). See Table 1 for a summary of all six individual cross-configuration comparisons. (b) Scatter plot of mean spike rate differences between the preferred and nonpreferred stimulus for the time period of 200–800 msec following physical alternation (resulting in monocular stimulation with either the preferred or nonpreferred stimulus). Each dot represents firing rates of a single neuron for the same population of cells as in a. Accordingly, CFG-A and CFG-B label the same comparisons between rival stimuli used in a. Response differences for physical alternation are typically larger than those obtained during flash suppression (for easier comparison, both plot axes are cropped at 40 Hz). Note the strong correlation of activity levels under this condition (r = 0.69, P < 0.001), indicating that the lack of correlation during flash suppression is not based on response differences to the monocular components chosen for comparison. (c) For the within-configuration comparison shown here, we divided up all trials of each of the four stimulus configurations and computed the average activity level for each of these data subsets. The high correlation (r = 0.75, P < 0.001) demonstrates the consistency of MT responses for repeated measurements during this task, thus ruling out trial-by-trial variability as the prime cause for the lack of correlation in a. Note that only the data between 0–40 imps per sec are shown.
Table 1.
Correlation of perceptual modulation across different stimulus configurations.
The independent modulation for different stimulus configurations can also be demonstrated by comparing the neuronal discrimination between alternate percepts following the conventions used in the computation of “choice probability” (15). Choice probability provides a measure of the statistical correspondence between the choice of an animal on each trial and the corresponding neural response on that trial [the exact value of “choice probability” for a particular neuron has been shown to vary with the type of task or training history (16–18)]. More generally, individual neural responses can be classified on any basis, and the ability of a cell to discriminate between two or more categories can be evaluated by using receiver operating characteristics (ROC) analysis. In the present case, responses were divided according to the alternate perceptual states imposed during BRFS. The results are shown in Fig. 4a and clearly demonstrate that individual neurons are uncorrelated in their ability to discriminate different pairs of motion percepts. Finally, the fact that different neurons show perceptual modulation with different stimulus configurations leads to the potentially important observation that a very high proportion of neurons can participate in reporting the perceptual outcome of a sensory conflict. The data in Fig. 4b demonstrate that when only four different stimulus configurations are considered, >90% of the neurons tested showed such modulation in at least one configuration. This proportion far exceeds that reported previously in the literature for this area and serves to further emphasize that shifting populations of neurons contribute to the perceptual solution of different bistable patterns.
Fig. 4.
Different rivalry stimuli reveal uncorrelated perceptual modulation among a large fraction of feature-selective neurons. (a) ROC analysis. Scatter plot and histograms of the mean value of the area under the ROC curve (AUC) for the percept-related activity difference for the same neurons and different stimulus configurations as in Fig. 3a. As indicated by a red dashed line, the mean value of AUC for each of the two stimulus classes amounts to 0.62 (0.619 and 0.168, accordingly). Nonetheless, we found no significant correlation between the AUC values of each neuron when the different stimulus configurations were compared (r = 0.22, P = 0.06). Furthermore, we found a small number of neurons that showed significant choice probabilities <0.5 for a unique stimulus configuration. Hence, this effect of reversed selectivity during rivalry stimulation as reported in previous studies (6, 10) seems to be stimulus-specific as well. Significance has been assessed by using a random permutation test with α = 0.05. The stimulus conventions and color scheme are similar to that used in Fig. 3. (b) Fraction of modulating neurons for a single-rivalry stimulus compared with testing with multiple stimulus sets. Only neurons that differentiated significantly between the rival stimuli in the physical alternation control condition were taken into analysis (data from monkey E00 by using random dot stimuli; n = 32). (Left) Average fraction of modulating neurons as assessed for each pair of random dot rival stimuli in isolation (see Fig. 1d; error bar is SEM). The result of ≈40% modulating neurons has been reported with different kinds of stimuli before and is considered typical of area MT. Note the rather small variability, which indicates that for each pair of rival stimuli the number of modulating neurons was largely comparable. (Right) Fraction of neurons that were significantly modulating with at least one pair of rival stimuli when all four stimulus configurations were considered together (Bonferroni corrected for multiple comparisons). Because different neurons exhibit significant perceptual modulation with different sets of stimuli (see Figs. 2b, 3a, and 4a), the overall number of cells with significant perceptual modulation is far larger than when a single stimulus pair is used for testing. In fact, the overall fraction of cells that modulate with different stimulus configurations is 93%, which reveals that almost all of the MT neurons we tested carried perceptual information with at least one stimulus configuration. A similar result was found when the grating stimuli were used (both monkeys, n = 85; 29% of neurons for a single stimulus vs. 74% when all stimulus configurations were considered together).
Discussion
Perceptual modulation, i.e., describing the changes in firing rate accompanying the resolution of sensory ambiguity or visual conflict, is a well documented property of some neurons in the visual cortex. This phenomenon is of great interest for the neurophysiologist because it indicates that the activity of feature-responsive cells draws at least partially upon internally generated, interpretive information about a sensory stimulus. Although flash suppression is not a “pure” perceptual stimulus because different perceptual states are preceded by differences in the order of stimulus presentation, it has been used successfully and contributed greatly to the understanding of how subjective mental states are supported in the visual brain (13, 19).
The present results argue that it is incorrect to classify individual neurons as belonging to a “sensory” or “perceptual” network because the same cell can appear to carry either type of signal depending upon the exact stimulus conflict. In fact, in our study, neurons often showed strong perceptual modulation for one rivalry configuration while showing none at all for another, even when the two configurations differed by having one rivaling monocular stimulus replaced by another that elicited the same sensory response. This condition frequently resulted in the same visual percept emerging despite grossly different patterns of underlying neural activity, an observation that might have important implications for understanding how the cortical machinery extracts and ultimately promotes one possible perceptual interpretation over another.
It is interesting to note that our results share several similarities with findings on the effects of visual selective attention on neuronal activity in area MT and other areas (20). In particular, the amount of percept-related modulation observed with bistable visual patterns resembles the amount of modulation found in endogenous attention tasks (3, 21, 22), although the type of stimulus competition is rather different (typically dichoptic stimuli in the case of rivalry vs. nonoverlapping, binocularly congruent stimuli in the case of attentional selection). Moreover, it has been found that the amount of attentional modulation can also be influenced by the choice of competing components of a stimulus pair (23). These similarities in neuronal processing can also be related to psychophysical interactions of selective attention and perceptual dominance (24), which might point to a common mechanism of endogenous stimulus selection during sensory processing (3).
One major implication of our results is that many more feature-responsive neurons in the visual cortex show perceptual modulation than previously appreciated when larger numbers of bistable stimuli are considered. In the present study, for example, ≈40% of MT neurons showed significant modulation with perception for any given stimulus (Fig. 4b). This finding is in agreement with previous studies (7, 9, 10). However, when considered across the four stimulus configurations, 70%–90% of cells showed modulation for at least one of the rivalry stimulus pairs. Thus, at least in this area, percept-modulated activity does not seem to be relegated to a specialized subclass of neurons, but is instead a shared feature of the majority of cells. It is likely that this percentage would increase even further if additional bistable stimuli were tested (this possibility is well supported by unpublished data, in which we have tested the same neurons with bistable structure-from-motion patterns). It is interesting to note that the repeated observation that 40%–50% of stimulus-tuned neurons show percept-related changes in MT might reflect the limits governing what fraction of feature-selective neurons can act at any one time to support a perceptual interpretation. It is also important to note that evidence from the present study (SI Fig. 7a), as well as from a previous one (16), indicates that the depth of perceptual modulation is, at least to some extent, related to the tuning strength of the cell. Clearly this relationship is only one factor influencing the perceptual modulation of a neuron with a give bistable pattern, and it is not sufficient to explain the idiosyncratic perceptual modulation demonstrated in the present study. Nonetheless, exploring further connections between the role of neurons during unambiguous and ambiguous visual patterns holds promise for gaining a more complete understanding of the role of individual feature-selective neurons in disambiguating a sensory conflict.
Materials and Methods
Subjects.
Electrophysiological recordings were performed in two healthy adult male rhesus monkeys (Macaca mulatta) weighing ≈14.5 and ≈9 kg, respectively. All experiments were carried out with great care to ensure the well being of the monkeys and were in full compliance with the guidelines of the local authorities (Regierungspräsidium Tübingen), as well as the European Community (EUVD 86/609/EEC) for the care and use of laboratory animals.
Neurophysiological Recordings.
At the beginning of each session, a Wheatstone-type mirror stereoscope was positioned in front of the monkeys' heads and calibrated while monkeys were performing a fixation task. After lowering electrodes into area MT, single units were isolated and receptive fields were manually mapped by using moving bars and gratings as stimuli. We tried to maximize the number of cells that were responsive for motion on the horizontal axis (i.e., left- or rightward motion) by lowering each electrode individually. After initial characterization, electrodes were left in place for the rest of the recording session. See SI Methods for additional details.
Behavioral Paradigm.
All trials started with a short period during which a fixation spot was shown in isolation (300–500 msec) and the monkey had to acquire and hold fixation. Fixation was defined as keeping the center of view within a fixation window of 0.5° of visual angle radius. No consistent eye movement patterns could be found to be associated with either perceptual state during both tasks. BRFS trials generally consisted of two parts. During a brief adapting period, one of the rivaling stimuli was presented monocularly, whereas a dark screen was shown to the other eye. After 850 msec, another stimulus was turned on (flashed) in the other eye. This sequence is known to lead to the second stimulus dominating perception following its presentation.
BRFS stimuli for monkeys K97 and E00 consisted of dissimilar drifting grating patterns presented dichoptically at the center of gaze. Gratings were of sinusoidal luminance profile, with maximum contrast provided by the screen. Their spatial frequency varied from one to five cycles per visual angle, and their temporal frequency varied one cycle per sec. Stimuli were adjusted to cover the receptive fields of a maximal amount of simultaneously recorded cells (between 2.75–4.5° of visual angle radius). Motion vectors of monocular stimuli were stepped between 0–360° in 45° increments. The size, spatial frequency, color, speed, and contrast of the stimuli were chosen to minimize partial suppression during the first second following flash suppression. The ocular configuration of the stimuli was pseudorandomized. Orthogonal drifting gratings shown to different eyes have been reported to occasionally evoke a percept of intermediate motion direction, between that of the two component patterns (25, 26). Although we have not observed this effect in a separate human psychophysical evaluation of our stimuli, we wanted to ensure that our results did not reflect such vector summation, which does not occur with drifting random dots (27), so we also produced binocular rivalry with random dots (monkey E00 only). The coherence of random dot patches was held constant at 100%. Reliable flash suppression with either type of stimuli was verified by several human observers (data not shown). See SI Methods for additional details.
Analysis.
All analyses were performed offline by using custom software written in MATLAB (The Mathworks Inc., Natick, MA). Single-unit activity was assessed offline by using a custom-written spike sorter. Spike density functions were computed by convolving each point in the poststimulus histograms with a Gaussian kernel of σ = 15 (28). Response levels for each condition corresponded to the average single-unit activity response for 200–800 msec after the second stimulus onset. The first 200 msec following stimulus onset were disregarded to focus on sustained firing rate differences, rather than any evoked activity transients. This time window was also used for ROC analysis for the assessment of perceptual modulation. Specifically, the value of the area under the ROC curve (AUC) was computed by using the approach described (15). Significant perceptual modulation was then assessed by means of a random permutation test by using Monte Carlo simulation with 4,000 repetitions. Details are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank J. Werner and A. Öltermann for technical assistance, Dr. D. Sheinberg for help with the stimuli, Dr. Y. Murayama for help with the acquisition software, M. Augath for conducting the anatomical MRI scans, Drs. K. Nielsen and C. Hofstötter for discussion, and Drs. M. Wilke, R. Kanai, and K. M. Müller for comments on an earlier version of the manuscript. This work was supported by the Max Planck Society.
Abbreviations
- AUC
area under the ROC curve
- BRFS
binocular rivalry flash suppression
- MT
medial temporal
- ROC
receiver operating characteristics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608489104/DC1.
References
- 1.Koch C. The Quest for Consciousness: A Neurobiological Approach. Greenwood Village, CO: Roberts & Company Publishers; 2004. [Google Scholar]
- 2.Blake R, Logothetis NK. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 3.Leopold DA, Logothetis NK. Trends Cogn Sci. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis NK. Philos Trans R Soc Lond B Biol Sci. 1998;353:1801–1818. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leopold DA, Maier A, Wilke M, Logothetis NK. In: Binocular Rivalry and Perceptual Ambiguity. Alais D, Blake R, editors. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- 6.Leopold DA, Logothetis NK. Nature. 1996;379:549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- 7.Dodd JV, Krug K, Cumming BG, Parker AJ. J Neurosci. 2001;21:4809–4821. doi: 10.1523/JNEUROSCI.21-13-04809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunewald A, Bradley DC, Andersen RA. J Neurosci. 2002;22:6195–6207. doi: 10.1523/JNEUROSCI.22-14-06195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley DC, Chang GC, Andersen RA. Nature. 1998;392:714–717. doi: 10.1038/33688. [DOI] [PubMed] [Google Scholar]
- 10.Logothetis NK, Schall JD. Science. 1989;245:761–763. doi: 10.1126/science.2772635. [DOI] [PubMed] [Google Scholar]
- 11.Sheinberg DL, Logothetis NK. Proc Natl Acad Sci USA. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gail A, Brinksmeyer HJ, Eckhorn R. Cereb Cortex. 2004;14:300–313. doi: 10.1093/cercor/bhg129. [DOI] [PubMed] [Google Scholar]
- 13.Kreiman G, Fried I, Koch C. Proc Natl Acad Sci USA. 2002;99:8378–8383. doi: 10.1073/pnas.072194099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe JM. Vision Res. 1984;24:471–478. doi: 10.1016/0042-6989(84)90044-0. [DOI] [PubMed] [Google Scholar]
- 15.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 16.Purushothaman G, Bradley DC. Nat Neurosci. 2005;8:99–106. doi: 10.1038/nn1373. [DOI] [PubMed] [Google Scholar]
- 17.Uka T, DeAngelis GC. Neuron. 2004;42:297–310. doi: 10.1016/s0896-6273(04)00186-2. [DOI] [PubMed] [Google Scholar]
- 18.Krug K, Cumming BG, Parker AJ. J Neurophysiol. 2004;92:1586–1596. doi: 10.1152/jn.00851.2003. [DOI] [PubMed] [Google Scholar]
- 19.Logothetis NK, Sheinberg DL. Annu Rev Neurosci. 1996;19:577–621. doi: 10.1146/annurev.ne.19.030196.003045. [DOI] [PubMed] [Google Scholar]
- 20.Treue S, Martinez Trujillo JC. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 21.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 22.Krug K. Philos Trans R Soc Lond B Biol Sci. 2004;359:929–941. doi: 10.1098/rstb.2003.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds JH, Chelazzi L, Desimone R. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell JF, Stoner GR, Reynolds JH. Nature. 2004;429:410–413. doi: 10.1038/nature02584. [DOI] [PubMed] [Google Scholar]
- 25.Cobo-Lewis AB, Gilroy LA, Smallwood TB. Spat Vis. 2000;13:415–429. doi: 10.1163/156856800741298. [DOI] [PubMed] [Google Scholar]
- 26.Andrews TJ, Blakemore C. Nat Neurosci. 1999;2:405–406. doi: 10.1038/8068. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Matthews N, Qian N. Vision Res. 2001;41:3639–3647. doi: 10.1016/s0042-6989(01)00217-6. [DOI] [PubMed] [Google Scholar]
- 28.Richmond BJ, Optican LM, Podell M, Spitzer H. J Neurophysiol. 1987;57:132–146. doi: 10.1152/jn.1987.57.1.132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.