Abstract
Micelles from the mixture of poly(ethylene glycol)-phosphatidyl ethanolamine conjugate (PEG-PE) and D-α-tocopheryl polyetheyene glycol 1000 succinate (TPGS) were prepared loaded with the poorly soluble anticancer drug camptothecin (CPT). The solubilization of CPT by the mixed micelles was more efficient than with earlier described micelles made of PEG-PE alone. CPT-loaded mixed micelles were stable upon storage and dilution and firmly retained the incorporated drug. The cytotoxicity of the CPT-loaded mixed micelles against various cancer cells in vitro was remarkably higher than that of the free drug. PEG-PE/TPGS mixed micelles may serve as pharmaceutical nanocarriers with improved solubilization capacity for poorly soluble drugs.
Keywords: Micelles, Mixed micelles, Polymer-lipid conjugates, TPGS, Poorly soluble drugs, Anticancer drugs, Camptothecin
1. Introduction
Currently, polymeric micelles are popular pharmaceutical nanocarriers for the delivery of poorly water-soluble drugs, which can be solubilized within the hydrophobic inner core of a micelle (Bader et al., 1984; Jones and Leroux, 1999). As a result, micelles can substantially improve solubility and bioavailability of various hydrophobic drugs (Lukyanov and Torchilin, 2004). The small size (10-100 nm) of micelles allows for the micelle efficient accumulation in pathological tissues with the permeabilized (“leaky”) vasculature, such as tumors and infarcts, via the enhanced permeability and retention (EPR) effect (Wu et al., 1993; Torchilin, 2001); in addition, this small size offers the advantage of simple sterilization of micelles by filtration through polycarbonate membranes with the cut off size of 0.2 μ m.
Among other polymeric micelles, micelles prepared from conjugates of polyethylene glycol (PEG) and diacyllipids, such as phosphatidylethanolamine (PE)-PEG-PE conjugates-have demonstrated high stability and low toxicity (Torchilin, 2002; Lukyanov et al., 2002). The very low CMC value of PEG-PE compounds (in a 10-5M range) indicates that PEG-PE micelles can preserve their integrity even upon the dilution in the blood pool during the supposed therapeutic application (Lukyanov and Torchilin, 2004). In our previous studies, we have successfully encapsulated a number of poorly soluble anticancer drugs into PEG-PE micelles with high efficiency and stability (Gao et al., 2002; Mu et al., in press).
Camptothecin (CPT), a plant alkaloid from Camptotheca acuminate (Wall et al., 1966) demonstrated strong antitumor activity against lung, ovarian, breast, pancreas, and stomach cancers (Giovanella and Cheng, 1991; Wall and Wani, 1995) by targeting intracellular topoisomerase I, a nuclear enzyme that reduces the torsional stress of supercoiled DNA (Hsiang and Liu, 1988; Garcia-Carbonero and Supko, 2002). However, CPT is insoluble in water (Hatefi1 and Amsden, 2002) and exists in two forms depending on the pH value: the active lactone forms at pH below 5 and the inactive open ring-carboxylated CPT-Na+ form, which present at neutral pH values (Wall, 1969; Fassberg and Stella, 1992). Although the lactone form of CPT is crucial for its anticancer activity, at physiological pH values most CPT molecules exist in the inactive carboxylate form. Thus, sparing solubility and labile lactone ring hinder the clinical application of CPT.
Various formulation strategies have been designed to increase the solubility of CPT, stabilize the lactone ring, and reduce the systemic toxicity of this drug, which involve solid lipid nanoparticles (Yang et al., 1999), microparticles (Shenderova et al., 1997), and liposomes (Tardi et al., 2000). Polymeric micelles also can effectively solubilize and stabilize CPT (Cortesi et al., 1997; Barreiro-Iglesiasa et al., 2004; Opanasopit et al., 2004), and enhance the antitumor efficiency of the encapsulated CPT due to the spontaneous accumulation in tumor tissues via the EPR effect (Maeda et al., 2000; Jain, 1997). CPT incorporation into PEG-PE micelles recently described by us (Mu et al., in press), allowed for the preparations with significantly higher CPT concentrations than described earlier (Cortesi et al., 1997; Barreiro-Iglesiasa et al., 2004; Opanasopit et al., 2004).
One may expect that further increase in the efficiency of micellar solubilization of CPT might be achieved by using mixed micelles as shown for some other poorly soluble drugs (Krishnadas et al., 2003). Interestingly, D-α-tocopheryl polyetheyene glycol 1000 succinate (TPGS), a derivative of the natural Vitamin E (α-tocopherol) and polyetheyene glycol 1000 is used as solubilizer, absorption enhancer and a vehicle for lipid-based drug delivery formulations (Eastman, 1998; Mu and Feng, 2003). It was also reported that succinate esters of Vitamin E, such as RRR-α-tocopheryl succinate, behave as potent, pro-apoptotic agent selective for cancer cells (Yu et al., 2001; Neuzil et al., 2001). TPGS itself can hardly be used as micelle-forming material to carry CPT because of the relatively low CMC value (approximately 1.3 × 10-4 M for industrial grade TPGS (Eastman, 1998), which may result in micelle dissociation in the blood, however its lipophilic portion is relatively bulky, which might allow for better drug solubilization if TPGS is used as a component of the mixed micellar drug delivery system. One can hypothesize that mixed micelles made of TPGS and PEG-PE if used as CPT carriers may allow for the better drug encapsulation and high anticancer efficiency.
Here, we describe the preparation of CPT-loaded mixed PEG-PE/TPGS micelles, some of their properties and increased in vitro cytotoxicity against several tumor cell lines.
2. Materials and methods
2.1. Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEG-PE) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL) and used without further purification. Camptothecin (CPT) was purchased from Sigma-Aldrich (St. Louis, MO, USA). D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) was received as a gift from Eastman Co. (USA). All other reagents and components of buffer solutions were analytical grade preparations. Distilled and deionized water was used in all experiments.
2.2. Preparation of camptothecin-loaded micelles
CPT dissolved in acetonitrile adjusted with acetic acid was added to a chloroform solution of TPGS and PEG-PE (molar ratio of TPGS:PEG-PE was 2:1). Organic solvents were removed by rotary vacuum evaporation. The film formed was additionally freeze-dried in vacuum, then hydrated with a suitable amount of a 5 mM HEPES-buffered saline (HBS), incubated at 37°C for 30 min, and then sonicated for a few minutes. The resultant mixture was filtered through 0.2μm Nylon filter (Fisher Scientific, USA) to remove the non-incorporated crystalline CPT (Gao et al., 2002).
2.3. HPLC analysis of micelles
Size exclusion HPLC was used to analyze the micelle preparation (D-7000 based HPLC system equipped with diode array and fluorescence detectors, Hitachi, Japan). A size-exclusion Shodex® column (Shoko Co. Ltd., Japan) was used with HBS as mobile phase at a flow rate of 1.0 ml/min. Micelle peak was detected by UV detector at the wavelength of 220 nm.
2.4. Efficiency of CPT incorporation
The quantity of the micelle-incorporated CPT was also determined by HPLC using the analytical column Waters Spherisorb® 5μm ODS2, 4.6 mm × 250 mm Analytical Cartridge (Waters, Ireland). After filtration of the CPT-containing micelle preparation through the 0.2 m nylon filter, the micelles were diluted by 200-300-fold with acetonitrile (to destroy micelles and release CPT), and an aliquot of the diluted solution was injected into HPLC system. The determination of the micellar CPT was performed using the calibration curve of pure CPT. The mobile phase composed of 66% (v/v) acetonitrile (HPLC grade) and pure water (pH 5.0 adjusted with acetic acid) was used with a flow rate of 1.1 ml/min. CPT was detected by the fluorescence detector using an excitation wavelength of 360 nm and an emission wavelength of 430 nm.
2.5. Micelle size measurement
The micelle size measurements and size distribution analysis were performed by the dynamic light scattering (DLS) using a Coulter® N4-Plus Submicron Particle Sizer (Coulter Corporation, Miami, FL).
2.6. Micelle stability
Drug-loaded or free PEG-PE/TPGS micelles were stored at either 4 or 25 °C for time periods varying from few weeks up to 3 months, and the samples were monitored for the changes in particle size and drug content during the storage period. CPT-loaded micelles were also incubated in HBS buffer solution (pH 7.4) at 37 °C with shaking at the speed of 100 rpm for a few days and their size was also monitored.
2.7. CPT release from various micelle formulations at “sink” conditions in vitro
The in vitro CPT release from TPGS/PEG2PE micelles was investigated using a hydrotropic agent sodium salicylate to create pseudo-sink conditions in a limited volume similar to previous studies with paxlitaxel release from polymeric micelles (Mu et al., in press; Cho et al., 2004). First, the solubility of CPT in Na salicylate solutions was investigated to confirm the applicability of the approach. With this in mind, the excess of CPT was added to a capped vial containing a fixed volume of Na salicylate solution in water using various Na salicylate concentrations. The mixture was stirred for 24 h and then filtered through a 0.2μm nylon membrane. An aliquot of the sample was collected and diluted with acetonitrile, and the concentration of CPT was measured by HPLC as described above.
Further, the in vitro CPT release from various micelles was studied in an aqueous solution containing 1 M Na salicylate as a release medium. One millimeter of CPT-loaded micelles was introduced into a dialysis membrane bag, MWCO = 1000 Da (Spectrum Lab., CA, USA), and the end-sealed dialysis bag was incubated in 20 ml of 1 M sodium salicylate at 37° C. The release system was shaken at a speed of 100 rpm. At predetermined time intervals, 2 ml aliquots were withdrawn and replaced with an equal volume of the fresh 1 M Na salicylate. The concentration of CPT in the samples was measured by HPLC as described above with the correction for the volume replacement.
2.8. Cell cultures
Murine Lewis lung carcinoma cells (LLC1), human colorectal adenocarcinoma cells (HT-29), and human breast adenocarcinomas cells (MCF7), all from the ATCC (Manassas, VA) were grown in DMEM medium (Cellgro, Kansas City, MO) with 2 mM L-glutamine additionally supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin G, 0.25μg/ml amphotericin B, and 100μg/ml streptomicin. Cultures were maintained at 37 °C in a humidified 5% CO2 sterile incubator.
2.9. Cytotoxicity assay
For the cytotoxicity analysis with PEG-PE/TPGS micelles, cells were seeded at 5 × 103 of LLC cells, 20 × 103 of HT-29 cells, and 10 × 103 of MCF-7 cells per well of 96-well plates in four-replicates. After 24 h, the medium was exchanged for the medium containing increasing concentrations of each of the tested formulations-free drug alone, empty micelles and drug-loaded micelles in the range of 0-100 ng/ml of CPT and 0-150 μg/ml of PEG-PE/TPGS. The cells were reseeded with medium containing the listed formulations after 2 days, and after 4 days exposure to various formulations, the cell viability was determined using CellTiter 96AQ® Kit (Promega, Madison, WI) according to manufacturer’s instructions.
3. Results and discussion
3.1. Micelle size, stability and CPT incorporation
All prepared micelles, with or without CPT load, had the mean diameter of approximately 12 nm and size distribution from 6 to 20 nm. The single peak on HPLC profile (Fig. 1) confirmed the formation of CPT-loaded PEG-PE/TPGS mixed micelles (certain asymmetry of the baseline around the peak may be explained by too high micelle concentration used since it diminished with the dilution). The dilution of the micelle sample influenced only the amplitude of the HPLC peak, without affecting its position or shape, confirming the high stability of the mixed micelles. At 11 mM, concentration of mixed micelle components (TPGS:PEG2PE molar ratio 2:1, CMC values of both components and their mixture are within the 10-6to 10-5M range and well below micelle concentration) and with 1.2 wt% of CPT added to the mixture, the micelle suspensions were produced containing 30 ± 2.0μg of the drug per 1 ml of the micelle suspension. The mixed micellar system increased the solubilization of CPT by at least 50% compared to the micelles made of PEG-PE alone (Mu et al., in press). The resulting CPT solubility is also significantly higher than what was described in previous reports (Cortesi et al., 1997; Barreiro-Iglesiasa et al., 2004) for CPT solubilization in polymeric micelles made of polyoxyethylene-polyoxypropylene block copolymer (the maximum of 6.6 μg of CPT per 1 ml) or pluronic-g-poly(acrylic acid) copolymers (7 μg of CPT per 1 ml of the micellar suspension).
Fig. 1.
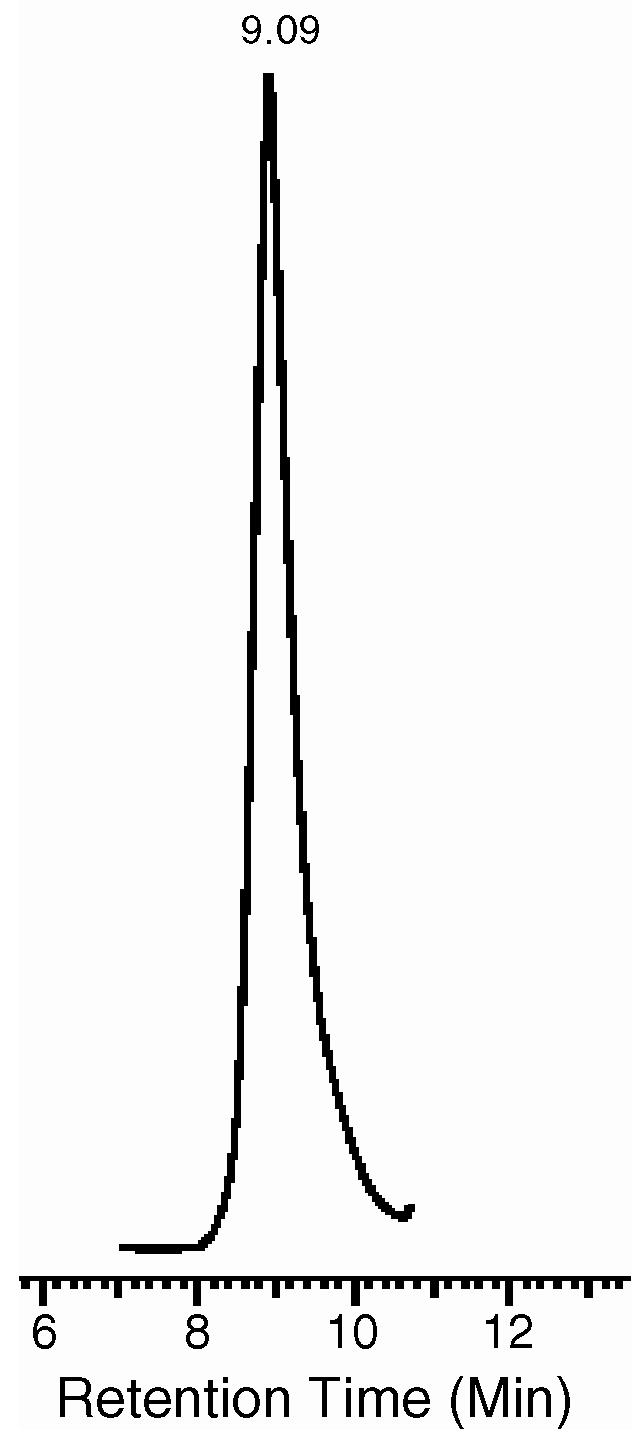
HPLC profile of CPT-loaded PEG-PE/TPGS micelles.
An enhanced CPT solubilization by mixed micelles compared to the micelles made of PEG-PE alone could be explained by an increased inner micelle core volume due to the bulky non-polar head of the TPGS molecule, which allows for more CPT to be encapsulated. At the quantities used, drug loading does not influence the micelle size significantly, indicating that the hydrophobic drug located in the hydrophobic core space of the micelles has almost no impact on the whole particle size.
Both drug-free and CPT-loaded micelle formulations did not show any noticeable changes in the particle size after 3 months at either room temperature or at 4°C. Fig. 2 presents the particle size of the CPT-loaded mixed micelles during 48 h incubation at 37 °C with shaking at the speed of 100 rpm. No significant changes in micelle size or size distribution were recorded, which supports the high stability of CPT-loaded PEG-PE/TPGS micelles.
Fig. 2.

PEG-PE/TPGS micelle size and size distribution pattern during incubation at 37 °C and 100 rpm shaking (A: before incubation; B: after 24 h incubation; C: after 48 h incubation).
3.2. CPT release from PEG-PE/TPGS micelles at sink conditions
The present work used the reported hydrotropic agent, 1 M Na salicylate, as a release medium (Mu et al., in press; Cho et al., 2004) to study the in vitro release of CPT from micellar formulation under sink condition. Preliminary, it was shown that the micelle integrity of CPT-loaded PEG-PE/TPGS mixed micelles was not affected by the Na salicylate solution up to 1 M concentration even upon a one week long co-incubation (Fig. 3). However, at higher Na salicilate concentrations, micelles destabilize and solubilize as follows from the gradual decrease in the micelle size till their full disappearance.
Fig. 3.

Mean diameters of CPT-loaded PEG-PE/TPGS micelles at various molar concentrations of Na salicylate in aqueous solution after one week long incubation.
The CPT release from PEG-PE and PEG-PE/TPGS micelles is presented in Fig. 4, which starts with an initial burst, followed by a very slow release phase. This release behavior reflects the CPT incorporation stability and can be explained through the geometry of CPT location within the micelles. The initial burst happened within the first few hours of incubation is attributed to the relatively small quantity of the drug associated on the interface of the micelle hydrophobic core and hydrophilic corona, or even within the micelle corona compartment and can proceed via both the hydration of the interfacial drug molecules and their passive diffusion. The drug incorporated into the inner core compartment stays firmly inside the micelles showing a very slow release even at sink conditions with 75-80% of the initially incorporated drug still being associated with the micelles even after 72 h incubation at 37°C.
Fig. 4.

Release profile of CPT from PEG-PE/TPGS micelles (▲) in 1.0 M aqueous Na salicylate at 37 °C; CPT-loaded micelles made of PEG-PE alone (□) were used for comparison.
It looks like mixed PEG-PE/TPGS micelle retain the drug even better than “pure” PEG-PE micelles indicating that the presence of TPGS facilitates not only CPT incorporation into micelles but also the stability of its entrapment, probably because of additional hydrophobic interactions of CPT molecules with the aromatic ring in TPGS non-polar head.
3.3. In vitro activity of CPT-containing micellar formulations
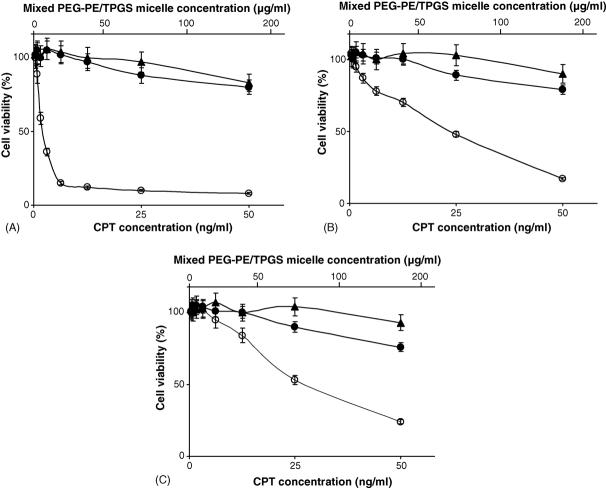
The in vitro cytotoxicity of CPT-loaded PEG-PE/TPGS micelles was investigated and compared with that of the free drug using murine LLC, human MCF7 and human HT-29 cells. As a negative control, empty mixed micelles were used. Cells were treated with the same concentrations of free or micellar CPT (as CPT). Empty micelles were added in the same concentrations as the tested CPT-micelles (same concentration of PEG-PE/TPGS micelle-forming material). After 4 days in the presence of micellar preparations, cells were analyzed for their survival using the MTS colorimetric assay for the dehydrogenase activity of viable cell. In all studied cell lines (Fig. 5), CPT in PEG-PE/TPGS micelles demonstrated a significantly superior cytotoxicity compared to that of the free drug. The IC50 values for various formulation towards LLC cells were 123 ± 12 ng/ml for free CPT (extrapolated) and only 2.1 ± 0.3 ng/ml for CPT-loaded PEG-PE/TPGS micelles; towards MCF7 cells these values were 229 ± 18 ng/ml for free CPT (extrapolated) and 29 ± 2.5 ng/ml for CPT-loaded PEG-PE/TPGS; towards HT-29 cells-294 ± 26 ng/ml for free CPT (extrapolated) and 31 ± 2.7 ng/ml for CPT-loaded PEG-PE/TPGS micelles. The cytotoxicity result could be explained by the enhanced solubility of the poorly soluble CPT in micelle solution, increased stability of the cytotoxic lactone form of CPT inside the micelle core, and better uptake of CPT-containing micelles by the cells. “Plain” PEG-PE were shown not to have any cytotoxicity against cancer cells (Mu et al., in press), whereas PEG-PE/TPGS mixed empty vehicles at used concentrations of the micelle-forming material, showed some inhibition of cell growth compared to that observed for the free CPT (tested in concentrations dramatically lower that that of the micelle-forming material) (Fig. 5). This could be explained by the reported cancer-specific cytotoxic property of TPGS to serve as a pro-apoptotic agent against cancer but not normal cells (Yu et al., 2001; Neuzil et al., 2001). In good agreement with that, PEG-PE/TPGS mixed empty micelle displayed no cytotoxicity, when tested against the H9c2 rat fetal cardiomyocytes used as a control non-cancerous cell line (data not shown).
Fig. 5.
In vitro cytotoxicity of free CPT (▲) and of CPT-free (•) and CPT-loaded (○ mixed PEG-PE/TPGS micelles against various cancer cell lines (A: LLC1 cells; B: MCF7 cells; C: HT29 cells).
4. Conclusion
Micelles from mixture of TPGS and PEG-PE conjugates (2:1 molar ratio) provide a more efficient solubilization of the poorly soluble anticancer drug, camptothecin (CPT), than the micelles made of PEG-PE alone. PEG-PE/TPGS mixed micelles firmly retain the incorporated CPT and demonstrate dramatically enhanced cytotoxicity against several tested murine and human cancer cell lines compared to the free drug. The enhanced cytotoxicity of CPT-loaded mixed micelles can be attributed to several factors: enhanced solubility of CPT in micelle solution, increased stability of the cytotoxic lactone form of CPT inside the micelle core, and better uptake of CPT-containing micelles by the cells. Mixed PEG-PE/TPGS micelles may represent a promising delivery platform for sparingly soluble anticancer drugs.
Acknowledgement
This work was supported by NIH Grant R01 EB001961 to Vladimir P. Torchilin.
References
- Bader H, Ringsdorf H, Schmidt B. Water soluble polymers in medicine. Angew. Makromol. Chem. 1984;123/124:457–485. [Google Scholar]
- Barreiro-Iglesiasa R, Brombergb L, Temchenkob M, Hattonb TA, Concheiroa A, Alvarez-Lorenzo C. Solubiliza-tion and stabilization of camptothecin in micellar solutions of pluronic-g-poly(acrylic acid) copolymers. J. Controlled Release. 2004;97:537–549. doi: 10.1016/j.jconrel.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Cho YW, Lee J, Lee SC, Huh KM, Park K. Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles. J. Controlled Release. 2004;97:249–257. doi: 10.1016/j.jconrel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cortesi R, Esposito E, Maietti A, Menegatti E, Nastruzzi C. Formulation study for the antitumor drug camptothecin: liposomes, micellar solutions and a microemulsion. Int. J. Pharm. 1997;159:95–103. [Google Scholar]
- Eastman Chemical Company, USA Oct, 1998. Publication EFC-226A.
- Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J. Pharm. Sci. 1992;81:676–684. doi: 10.1002/jps.2600810718. [DOI] [PubMed] [Google Scholar]
- Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyl-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–982. [Google Scholar]
- Garcia-Carbonero R, Supko JG. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin. Cancer Res. 2002;8:641–661. [PubMed] [Google Scholar]
- Giovanella BC, Cheng HR. Complete growth inhibition of human cancer xenografts in nude mice by treatment with 20-Scamptothecin. Cancer Res. 1991;51:3052–3055. [PubMed] [Google Scholar]
- Hatefi1 A, Amsden B. Camptothecin delivery methods. Pharm. Res. 2002;19:1389–1399. doi: 10.1023/a:1020427227285. [DOI] [PubMed] [Google Scholar]
- Hsiang YH, Liu LF. Identification of mammalian topoiso-merate I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722. [PubMed] [Google Scholar]
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 1997;26:71–90. doi: 10.1016/s0169-409x(97)00027-6. [DOI] [PubMed] [Google Scholar]
- Jones MC, Leroux JC. Polymeric micelles-a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Krishnadas A, Rubinstein I, Onyuksel H. Sterically stabilized phospholipid mixed micelles: in vitro evaluation as a novel carrier for water-insoluble drugs. Pharm. Res. 2003;20:297–302. doi: 10.1023/a:1022243709003. [DOI] [PubMed] [Google Scholar]
- La SB, Okano T, Kataoka K. Preparation and characterization of the micelle forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly (betabenzyl l-aspartate) block copolymer micelles. J. Pharm. Sci. 1996;85:85–90. doi: 10.1021/js950204r. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Gao Z, Mazzola L, Torchilin VP. Polyethylene glycol-diacyllipid micelles demonstrate increased accumulation in subcutaneous tumors in mice. Pharm. Res. 2002;19:1424–1429. doi: 10.1023/a:1020488012264. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv. Drug Deliv. Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- Mu L, Chrastina A, Levchenko T, Torchilin VP. Micelles from poly(ethylene glycol)-phosphatidyl ethanolamine conjugates (PEG-PE) as pharmaceutical carriers for poorly soluble drug camptothecin. J. Biomed. Nanotechnol. in press. [Google Scholar]
- Mu L, Feng S. PLGA/TPGS nanoparticles for controlled release of paclitaxel: effects of the emulsifier and the drug loading ratio. Pharm. Res. 2003;20:1864–1872. doi: 10.1023/b:pham.0000003387.15428.42. [DOI] [PubMed] [Google Scholar]
- Neuzil J, Weber T, Schroder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Nerge-Salvayer A, Sticha M, Coffey RJ, Weber C. Induction of cancer cell apoptosis by α-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403–415. doi: 10.1096/fj.00-0251com. [DOI] [PubMed] [Google Scholar]
- Opanasopit P, Yokoyama M, Watanabe M, Kawano K, Maitani Y, Okano T. Block copolymer design for camptothecin incorporation into polymeric micelles for passive tumor targeting. Pharm. Res. 2004;21:2001–2008. doi: 10.1023/b:pham.0000048190.53439.eb. [DOI] [PubMed] [Google Scholar]
- Shenderova A, Burke TG, Schwendeman SP. Stabilization of 10-hydroxycamptothecin in poly (lactide-co-glycolide) microsphere delivery vehicles. Pharm. Res. 1997;14:1406–1414. doi: 10.1023/a:1012172722246. [DOI] [PubMed] [Google Scholar]
- Tardi P, Choice E, Masin D, Redelmeier T, Bally M, Madden TD. Liposomal encapsulation of topotecan enhances anticancer efficacy in murine and human xenograft models. Cancer Res. 2000;60:3389–3393. [PubMed] [Google Scholar]
- Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J. Controlled Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv. Drug Deliv. Rev. 2002;54:235–252. doi: 10.1016/s0169-409x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- Wall ME, Wani MC, Cook CE, Palmer KH, McPhail HT, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966;88:3888–3890. [Google Scholar]
- Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic. Cancer Res. 1995;55:753–760. [PubMed] [Google Scholar]
- Wall ME. In: Mothes K, Schreiber K, Schutte HR, editors. Alkaloids with antitumor activity; International Symposium on Biochemistry and Physiology of the Alkoloids; Academie-Verlag, Berlin. 1969.p. 77. [Google Scholar]
- Wu NM, Da D, Rudoll TL, Needham D, Whorton AR, Dehirst MW. Increased microvascular permeability contributes to preferential accumulation of steath liposomes in tumor tissue. Cancer Res. 1993;53:3765–3770. [PubMed] [Google Scholar]
- Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenous injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Controlled Release. 1999;59:299–307. doi: 10.1016/s0168-3659(99)00007-3. [DOI] [PubMed] [Google Scholar]
- Yu W, Liao QY, Hantash FM, Sanders BG, Kline K. Activation of extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-α-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001;61:6569–6576. [PubMed] [Google Scholar]