Although we fully agree with Michael Sgro and colleagues regarding the need for early identification and effective management of neonatal hyperbilirubinemia, we were surprised to see their recommendation that the Coombs' test be used to screen for hyperbilirubinemia in all infants born to mothers with type O blood.1 Although the Coombs' or direct antibody test (DAT) is an important test when trying to identify the cause of neonatal hyperbilirubinemia, recent studies have shown that it has extremely limited usefulness in predicting the development of significant hyperbilirubinemia.
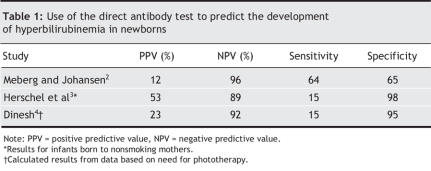
The DAT has been shown to have a positive predictive value of 12%–53% and a sensitivity of 15%–64% for the subsequent development of hyperbilirubinemia, which limits its usefulness as a screening test (Table 1).2–4Herschel and colleagues found that in comparison with measurements of end-tidal carbon monoxide concentration, the DAT showed only 8.5% sensitivity, 97.6% specificity and a positive predictive value of 25% for the detection of hemolysis in neonates.3
Table 1
In addition to having very low predictive ability, the DAT is costly when used as a screening test. US studies of the costs of evaluating neonatal jaundice have reported the cost per test to be US$17-$47.2,5,6
Newman and colleagues concluded that the investigation of hyperbilirubinemia should be individualized, with more aggressive investigation of infants with early onset or severe hyperbilirubinemia.6 Holtzman has also stressed the need for critical appraisal of strategies intended to identify infants with hyperbilirubinemia.7
In Calgary, routine DAT testing is being phased out in favour of a comprehensive hospital- and community-based transcutaneous bilirubinometry program. We believe that it provides a convenient, rapid, painless, cost-effective and accurate screening assessment for hyperbilirubinemia in the term and near-term neonate, particularly when incorporated into routine well-baby visits by public health nurses.8
We believe that the DAT should be reserved for diagnostic purposes in children with early or clinically significant hyperbilirubinemia.
REFERENCES
- 1.Sgro M, Campbell D, Shah V. Incidence and causes of severe hyperbilirubinemia in Canada. CMAJ 2006;175(6):587-90. [DOI] [PMC free article] [PubMed]
- 2.Meberg A, Johansen KB. Screening for neonatal hyperbilirubinemia and ABO alloimunization at the time of testing for phenylketonuria and congenital hypothyreosis. Acta Paediatr 1998;87:1269-74. [DOI] [PubMed]
- 3.Herschel M, Karrison T, Wen M, et al. Evaluation of the direct antiglobulin (Coombs') test for identifying newborns at risk for hemolysis as determined by end-tidal carbon monoxide concentration (ETCOc); and comparison of the Coombs' Test with ETCOc for detecting significant jaundice. J Perinatol 2002;22:341-7. [DOI] [PubMed]
- 4.Dinesh D. Review of positive direct antiglobulin tests found on cord blood sampling. J Paediatr Child Health 2005;41(9-10):504-7. [DOI] [PubMed]
- 5.Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics 2004;114:917-24. [DOI] [PubMed]
- 6.Newman TB, Easterling MJ, Goldman ES. Laboratory evaluation of jaundice in newborns: frequency, cost and yield. Am J Dis Child 1990;144:364-8. [DOI] [PubMed]
- 7.Holtzman NA. Management of hyperbilirubinemia: quality of evidence and cost. Pediatrics 2004;114:1086-8. [DOI] [PubMed]
- 8.Engle WD, Jackson GL, Stehel EK, et al. Evaluation of a transcutaneous jaundice meter following hospital discharge in term and near-term neonates. J Perinatol 2005;25:486-90. [DOI] [PubMed]