Abstract
Collagen biosynthesis in both invertebrates and vertebrates is critically dependent upon the activity of lysyl hydroxylase (LH) enzymes. In humans, mutations in the genes encoding LH1 and LH2 have been shown to cause two distinct connective tissue disorders, Ehlers-Danlos (Type VIA) and Bruck syndromes. While the biochemical properties of these enzymes have been intensively studied, their embryonic patterns of expression and developmental roles remain unknown. We now present the cloning and analyses of the genes encoding LH1 and LH2 in the zebrafish, Danio rerio. We find these genes to be similarly organized to other vertebrate lh (plod) genes, including the presence of an alternatively spliced exon in lh2. We also examine the mRNA expression patterns of lh1 and lh2 during embryogenesis and find them to exhibit unique and dynamic patterns of expression. These results strongly suggest that LH enzymes are not merely housekeeping enzymes, but play distinct developmental roles. The identification of these genes in the zebrafish, a genetic model organism whose development is well characterized, now provides the basis for the establishment of the first animal models for both Ehlers-Danlos (Type VIA) and Bruck syndromes.
Keywords: zebrafish, lysyl hydroxylase, lh, plod, extracellular matrix, collagen
1. Introduction
Members of the lysyl hydroxylase (LH) enzyme family catalyze the addition of hydroxyl groups to lysine residues in collagens and other proteins containing the sequence X-Lys-Gly (for review, see Myllyharju and Kivirikko, 2004). These hydroxylysine residues serve as sites of attachment for carbohydrate chains and also participate in the formation of intermolecular crosslinks (reviewed in Risteli et al., 2004). To date, three members of the LH family have been identified in vertebrates. Although the enzymes do not appear to require strict sequence specificity for substrate recognition, they do have distinct biological roles (Risteli et al., 2004; Wang et al., 2000). LH1 specifically hydroxylates lysine residues in regions of substrate proteins that form triple helices (Steinmann et al., 1995). In humans, mutations in lh1 (also known as plod1) have been linked to Ehlers-Danlos (type VIA) syndrome, a recessive disorder characterized by hyperextendable skin, joint hypermobility and kyphoscoliosis (Yeowell and Walker, 2000). In contrast to LH1, LH2 also has telopeptidyl lysyl hydroxylase activity (van der Slot et al., 2003). Recently, mutations in human lh2 (plod2) were linked to Bruck syndrome, distinguished by fragile bones and contractures of the large joints (Ha-Vinh et al., 2004; van der Slot et al., 2003). LH3, in addition to exhibiting lysyl hydroxylase activity, is the only LH enzyme to possess additional glycosyltransferase activities that serve to further modify hydroxylysine residues to galactosylhydroxylysine and glucosylgalactosylhydroxylysine (Heikkinen et al., 2000; Rautavuoma et al., 2002; Wang et al., 2002). Although mutations in human lh3 (plod3) have not yet been identified, a mouse knockout reveals this gene has an embryonic lethal phenotype and is required for type IV collagen secretion (Rautavuoma et al., 2004; Ruotsalainen et al., 2006). More recently, we have demonstrated that diwanka, the zebrafish homolog of lh3, plays a specific role in motor axon migration (Schneider and Granato, 2006).
Although LH enzymes are recognized to play a key step in collagen biosynthesis, little is known regarding their developmental roles. While the tissue distribution of lh gene expression has been examined by Northern blot analysis, with the exception of lh3, there have been no published accounts of the in situ expression patterns of these genes during embryogenesis in any vertebrate species (Passoja et al., 1998b; Rautavuoma et al., 2004; Ruotsalainen et al., 1999; Valtavaara et al., 1997; Valtavaara et al., 1998; Yeowell et al., 1994; Yeowell and Walker, 1999). In this paper, we report the cloning of zebrafish lh1 and lh2 and their patterns of expression throughout the first two days of development. We find that both genes have organizational structures similar to other vertebrate lh genes, and that, as in other vertebrates, lh2 exhibits alternative splicing. For the first time in any vertebrate species, we show the expression patterns of these genes during embryogenesis, and demonstrate that they display highly restricted and dynamic patterns of expression. Furthermore, we observe that lh1 and lh2 mRNAs are found in both unique and overlapping domains, indicative of their having distinct developmental roles. We discuss how these findings will provide the basis for future in vivo analyses of the roles of lh genes in development and disease.
2. Results
2.1 Cloning of zebrafish lh1 and lh2
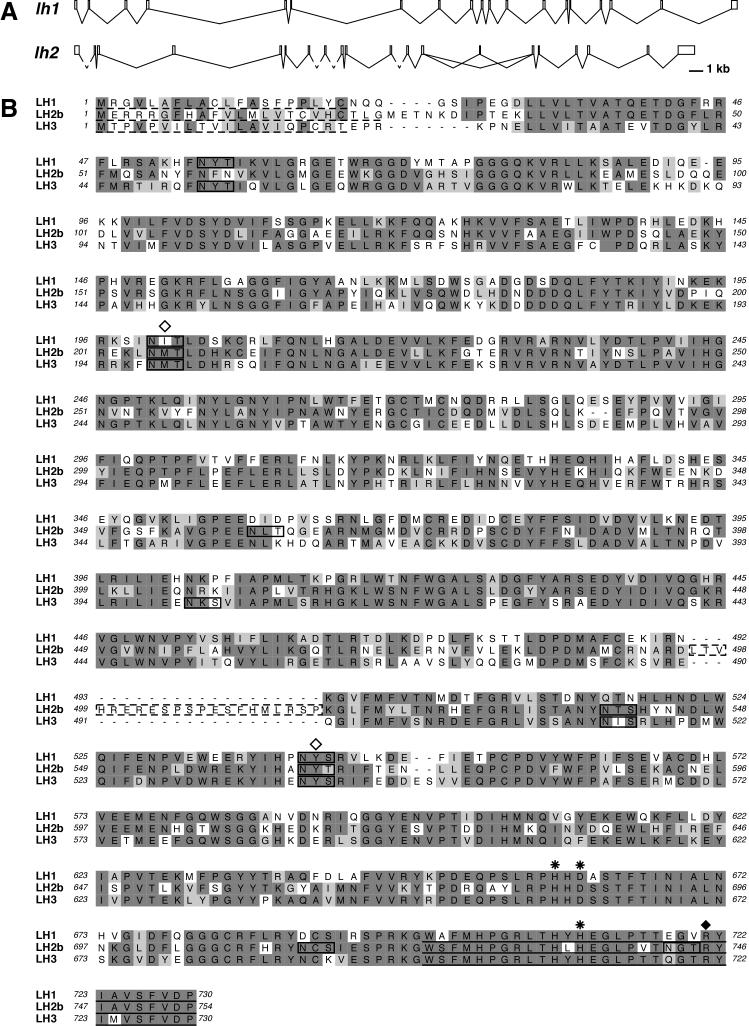
We identified several zebrafish cDNA clones whose sequences were homologous to portions of other vertebrate lh1 genes (see Experimental Procedures for details of cloning). Further analysis led to the identification of the full-length zebrafish lh1 cDNA sequence (Genbank DQ020093). A subsequent BLAST analysis of the zebrafish genome assembly in Ensembl (http://www.ensembl.org/Danio_rerio/index.html) with this cDNA sequence identified the complete genomic sequence corresponding to lh1 (Zv6_scaffold1312). SIM4 was used to predict the intron-exon boundaries of lh1 (http://pbil.univ-lyon1.fr/sim4.php) and revealed 19 exons, whose sizes correspond closely to those of human lh1, as well as zebrafish lh3 (Fig. 1A and Table 1). Consistent with the lack of Alu elements (present in several introns of human lh1) in the zebrafish genome, there is no conservation of intron size (Fig. 1A and Izsvak et al., 1997; Yeowell and Walker, 2000). Conceptual translation of zebrafish lh1 predicts a 730 amino acid (aa) protein with a 20 aa signal sequence (Fig. 1B). ClustalX analysis shows this protein exhibits 67% identity to human LH1, but only 59% identity to zebrafish LH3. There are three potential N-linked glycosylation sites (NXS/T) in zebrafish LH1. Two of these sites are also conserved in other vertebrate LH1 proteins and their glycosylation has been shown to contribute to lysyl hydroxylase activity (Fig. 1B, Pirskanen et al., 1996). All amino acid residues demonstrated to be required for co-factor binding by other vertebrate LH proteins are conserved in zebrafish LH1 (Fig. 1B, Passoja et al., 1998a; Pirskanen et al., 1996).
Figure 1.
Comparison of zebrafish lh genes. A: Genomic organization of zebrafish lh1 and lh2. The dashed lines in lh2 represent intron sequences whose lengths could not be determined due to the lack of available genomic sequence. B: ClustalW was used to align the proteins encoded by the zebrafish lh1, lh2 and lh3 genes. In LH2b, the dashed box indicates the residues encoded by the alternatively spliced exon 13A. Identical residues are shown in dark gray, similar residues in light gray. Dashed line: predicted signal sequence. Solid line: putative ER retention signal (Suokas et al., 2003). Boxes indicate potential sites for N-glycosylation. Open diamond: N-glycosylation site required for full enzymatic activity of human LH1 (Pirskanen et al., 1996). Asterisk: Fe2+ binding site (Pirskanen et al., 1996). Closed diamond: 2-oxogluatarate binding site (Passoja et al., 1998a).
Table 1.
Comparison of lh gene exon lengths
| Exon Number |
Dr lh1 (bp) |
Hs lh1 (bp)a |
Dr lh2 (bp) |
Mm lh2 (bp)b |
Dr lh3 (bp)c |
|---|---|---|---|---|---|
| 1 | 139 | 132 | 291 | 308 | 193 |
| 2 | 92 | 92 | 92 | 92 | 92 |
| 3 | 134 | 134 | 137 | 137 | 137 |
| 4 | 164 | 164 | 164 | 164 | 164 |
| 5 | 113 | 113 | 113 | 113 | 113 |
| 6 | 64 | 64 | 64 | 64 | 64 |
| 7 | 98 | 98 | 98 | 98 | 98 |
| 8 | 102 | 102 | 102 | 102 | 102 |
| 9 | 132 | 132 | 126 | 126 | 132 |
| 10 | 122 | 122 | 122 | 122 | 122 |
| 11 | 105 | 105 | 105 | 105 | 105 |
| 12 | 126 | 126 | 126 | 126 | 126 |
| 13 | 142 | 142 | 142 | 142 | 142 |
| 13Ad | -- | -- | 63 | 63 | -- |
| 14 | 114 | 114 | 114 | 114 | 114 |
| 15 | 66 | 66 | 66 | 66 | 72 |
| 16 | 105 | 105 | 105 | 105 | 105 |
| 17 | 147 | 147 | 147 | 147 | 147 |
| 18 | 126 | 126 | 126 | 126 | 126 |
| 19 | 591 | 887 | 1074 | 1347 | 419 |
Heikkenen et al. (1994)
Ruotsalainen et al. (2001)
alternatively spliced in lh2
bp: base pairs, Dr: Danio rerio, Hs: Homo sapiens, Mm: Mus musculus
We also identified cDNA clones whose sequences exhibited homology to portions of vertebrate lh2 genes. Studies of lh2 in other species show that this gene, in contrast to lh1 and lh3, exhibits alternative splicing. Consistent with these studies, we identified cDNAs corresponding to the two known lh2 splice variants in the course of cloning full-length zebrafish lh2 (Genbank DQ020094 (lh2a) and DQ020095 (lh2b); see Experimental Procedures for details). BLAST analysis of the zebrafish genome assembly in Ensembl revealed that the corresponding lh2 genomic sequence is currently distributed between two contigs (Zv6_scaffold3453 and Zv6_scaffold2680). SIM4 analyses of these sequences predict that zebrafish lh2a contains 19 exons, whereas zebrafish lh2b contains an additional exon (13A) of 63 bp (Fig.1 and Table 1). Like lh1, exon sizes (but not intron sizes) in zebrafish lh2 closely match those of other vertebrate lh2 genes, as well as those of zebrafish lh3 (Fig. 1A and Table 1). Conceptual translation predicts lh2 to encode 733 (LH2a) and 754 aa (LH2b) proteins, which have 23 aa signal sequences and five potential N-glycosylation sites (Fig. 1B). These proteins exhibit 70% identity to human LH2a/LH2b, but only 59% identity to other zebrafish LH proteins.
2.2 Analysis of lh2 splice variants
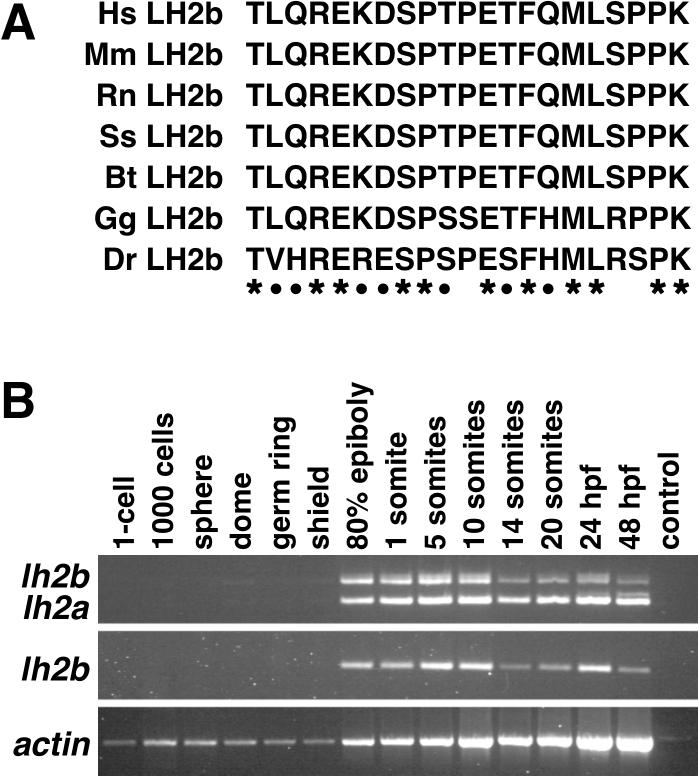
To date, all lh2 mutations identified in Bruck syndrome, a disorder associated with decreased levels of telopeptidyl lysyl hydroxylase (TLH) activity, are predicted to affect both splice variants of this gene (Ha-Vinh et al., 2004; van der Slot et al., 2003). However, only increased levels of lh2b mRNA expression appear to be associated with increased levels of TLH activity found in fibrotic lesions, suggesting that the sequence of exon 13A encodes the LH2 domain responsible for specifying TLH activity (van der Slot et al., 2003; van der Slot et al., 2004). We aligned the amino acid sequence encoded by exon 13A of zebrafish lh2 to that of other vertebrate species (obtained from GenBank) to identify residues whose evolutionary conservation might indicate their contribution to this activity (Fig. 2A). We observed a 90% degree of conservation to human LH2b in this region, higher than the 83% conservation of the full-length proteins. We found that 11 of the 21 amino acids encoded by exon13A were invariant in the seven species examined, and an additional 7 showed only conservative changes. The high degree of conservation in these amino acids is strongly suggestive of this domain contributing to the TLH activity associated with LH2b. Further analyses will be required to determine the importance of each amino acid to this activity.
Figure 2.
Analyses of lh2 splice variants. A: Alignment of the amino acid sequence encoded by exon 13A of various vertebrate lh2 genes. Hs: Homo sapiens (NP_891988), Mm: Mus musculus (AAK20118), Rn: Rattus norvegicus (XP_579707), Ss: Sus scrofa (ABB02507), Bt: Bos taurus (XP_613110), Gg: Gallus gallus (XP_422695), Dr: Danio rerio. Asterisks indicate residues having 100% identity; closed circles show residues exhibiting conserved changes. B: RT-PCR analysis of zebrafish lh2 splice variant expression at various stages of embryonic development. Top row: amplification of both lh2b (top) and lh2a (bottom) transcripts with primer pair A (see Experimental Procedures). Middle row: unique amplification of lh2b transcripts with primer pair B. Note the decrease in lh2b expression at the 14 somite stage detected with both primer pairs. Bottom row: amplification of actin transcripts (control).
To determine whether there are differences in the temporal expression of the lh2 splice variants, we performed RT-PCR on cDNA prepared from zebrafish embryos at various stages (Fig. 2B), using primers that distinguished these cDNAs. These studies indicate that concomitant expression of both splice variants begins during gastrulation and persists through 48 hours post fertilization (hpf), the latest stage examined. While lh2a mRNA levels remain relatively constant throughout this period, lh2b expression appears to decrease slightly during later stages of somitogenesis, increase at 24 hpf and then decline again at 48 hpf. These finding suggest that, although generally coordinately expressed, lh2a and lh2b transcripts may also be independently regulated.
2.3 Expression patterns of lh1 and lh2 during embryonic development
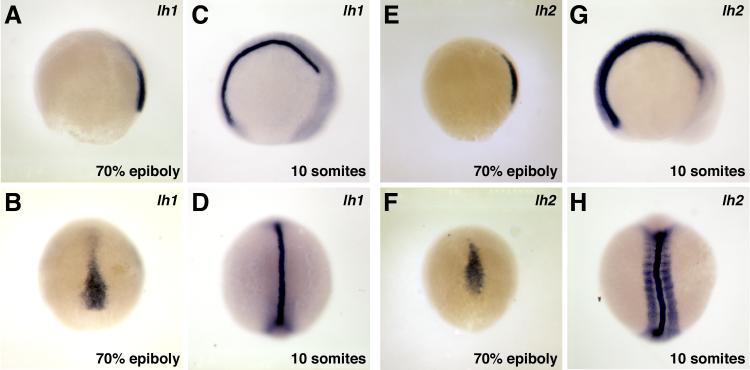
We performed whole mount in situ hybridization to determine the expression patterns of lh1 and lh2 during embryogenesis. Because there is only a 63 bp difference between the lh2 splice variants, the lh2 in situ probe used is unlikely to distinguish between the two transcripts. Thus, the expression patterns shown may reflect either or both lh2 splice variants. Consistent with the RT-PCR data, expression of lh1 and lh2 was first detected by in situ hybridization during gastrulation. During these stages, expression of both genes was confined to the axial mesoderm, the patterns being essentially indistinguishable from one another (Fig. 3). Differences in lh1 and lh2 expression patterns were first detected during somitogenesis. Through out these stages, lh1 expression was restricted to the notochord, while additional domains of lh2 expression were observed in the hypochord, floorplate and myotome (Figs. 3 and 4).
Figure 3.
Expression of lh1 and lh2 at early developmental stages. A-D: lh1, E-H: lh2. A, B, E, F: lh1 and lh2 are both expressed only in axial mesoderm during gastrulation (A, E: lateral views, B, F: dorsal views). C, D, G, H: During somitogenesis, lh1 expression is restricted to the notochord, while an additional domain of lh2 expression is observed in the somites and presomitic mesoderm (C, G: lateral views, D, H: dorsal views).
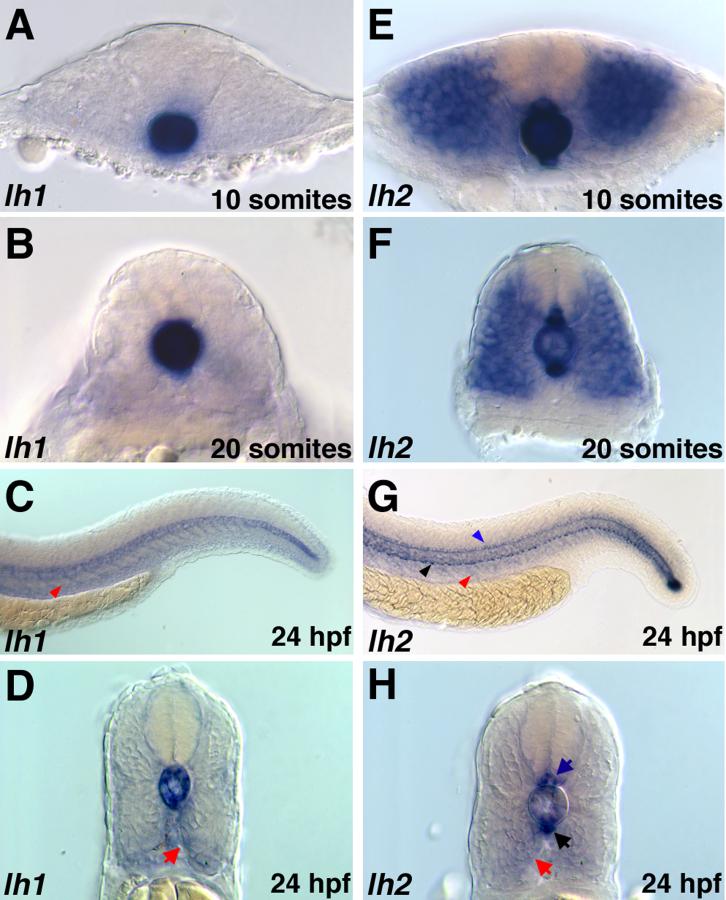
Figure 4.
Expression of lh1 and lh2 mRNAs in the trunk region. A-D: lh1, E-H: lh2. AB: Cross-sectional views showing restriction of lh1 expression to the notochord throughout the period of somitogenesis. E-F: Cross-sectional views demonstrating maintenance of lh2 in the notochord, myotome, floor plate and hypochord during somitogenesis stages. C, D: At 24 hpf, lh1 expression in the notochord is reduced and low levels of expression are observed in the region of the developing sclerotome (red arrows) along the medial surface of the ventral somite (C: lateral view, D: cross sectional view) E, F: At 24 hpf, expression of lh2 in the notochord and myotome is downregulated, while expression in the hypochord (black arrows) and floor plate (blue arrows) persists. Weak expression in the region of sclerotome (red arrow) is also detected (G: lateral view, H: cross sectional view).
Beginning at 24 hpf, the expression patterns of lh1 and lh2 shift again. As lh1 expression in the notochord diminishes, a new domain of low-level expression appears in the sclerotomal compartment of the ventral somite (Figs. 4C, D). Furthermore, otic vesicle expression of lh1, only weakly detectable at 20 somites, intensifies (Fig. 5A). A close examination of the developing ear at this stage reveals this expression is restricted to the medial surfaces of the epithelial cells lining the otic vesicle and is more concentrated in the anterior region. lh2 mRNA expression at 24 hpf, like that of lh1, is downregulated in the notochord and weakly upregulated in the region of the developing sclerotome (Figs. 4G, H). Furthermore, the robust myotomal lh2 expression that was observed throughout somitogenesis disappears, though expression in the floor plate and hypochord remains strong (Figs. 4G, H). In contrast to lh1, however, there is no detectable expression of lh2 in the otic vesicle (data not shown).
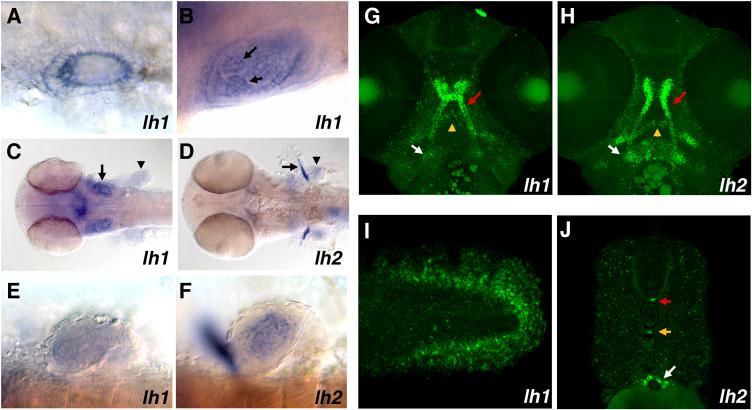
Figure 5.
Tissue specific expression of lh1 and lh2 mRNAs. A, B: Dorsal views of lh1 expression in the otic vesicle at 24 (A) and 48 (B) hpf (anterior at right). Arrows indicate expression in the epithelial projections of the developing semicircular canals. C, D: dorsal views of 48 hpf embryos showing lh1 (C) and lh2 (D) expression in the anterior portion of the embryo. Expression of both mRNAs can be seen in the head and developing pectoral fins (arrowheads). Only lh1 is expressed in the otic vesicles (arrow in C), whereas lh2 is uniquely expressed in the cleithra (arrow in D). E, F: Higher magnification lateral views of lh1 (E) and lh2 (F) expression in the pectoral fins at 48 hpf. In F, the cleithrum is out of focus. Note the more intense central staining of lh2 as compared to lh1. G, H: Confocal stacks showing lh1 (G) and lh2 (H) expression in head cartilages at 48 hpf (ventral views, anterior at top). Both mRNAs are strongly expressed in the neurocranium (red arrow), while lh2 expression in the developing mandibular (yellow arrow) and hyoid arches (white arrow) is more pronounced than that of lh1. I: Lateral view of lh1 expression in the tail at 48 hpf. Expression is restricted to the developing actinotrichia. J: Cross-sectional view of lh2 mRNA expression at 48 hpf. Expression in the floor plate (red arrow) and hypochord (yellow arrow) persists at these stages. Transcripts can also be detected in the developing intestine (white arrow).
As development proceeds, the domains of lh1 and lh2 expression remain distinct and continue to evolve. By 48 hpf, notochordal expression of lh1 has ceased. However, a new domain of expression is observed at the posterior tip of the tail in the developing collagenous actinotrichia, which provide strength to the fin folds (Fig. 5I). lh1 continues to be expressed in the otic vesicle, where it now can be detected in the epithelial projections of the developing semicircular canals (Fig. 5B, C). In the head of the embryo, lh1 expression is strongly expressed in the developing neurocranium and more weakly in the mandibular and hyoid arches (Fig. 5C, G). An additional domain of lh1 expression is observed in the pectoral fin buds, where its mRNA is present throughout the mesenchymal core of the developing fin, but absent from the overlying epithelium (Fig. 5C, E). At 48 hpf, lh2 expression is also observed in the mesenchymal region of the pectoral fin buds, though it appears to be more concentrated in the central-most precartilagenous core than lh1 (Fig. 5C, F). There is also robust expression of lh2 in the developing cleithra, the bones that will connect the pelvic girdle to the skull (Fig. 5D). Within the head, high levels of lh2 expression are found in condensing cartilage of both the mandibular and hyoid arches, in addition to the neurocranium (Fig. 5D, H). Along the length of the tail, lh2 expression in the floor plate and hypochord persists, while a new domain of expression is observed in the developing intestine.
3. Discussion
Collagen biosynthesis is a multi-step process, requiring the coordinated activity of several different modifying enzymes (reviewed in Myllyharju and Kivirikko, 2004). Among these are the ER-resident lysyl hydroxylases, which perform co-translational modifications of lysine residues in proteins bearing the sequence X-Lys-Gly. Although the biochemical reactions they perform have been the subject of many in vitro studies, there is still much to be learned about the in vivo requirement for these enzymes, particularly during the period of embryonic development. In this paper, we have presented the cloning, sequence analysis and embryonic expression patterns of zebrafish lh1 and lh2.
The zebrafish lh1 and lh2 genes have organizations similar to their human counterparts and, based on protein sequence similarities, are expected to exhibit comparable enzymatic activities. Though LH-mediated modifications are known to be important for collagen fibril assembly and intermolecular interactions, the critical in vivo requirement for these enzymes is underscored by the finding that, in humans, mutations in lh1 and lh2 result in Ehlers-Danlos (type VIA) and Bruck syndromes, respectively (Ha-Vinh et al., 2004; Risteli et al., 2004; van der Slot et al., 2003; Yeowell and Walker, 2000). Thus, our identification of the zebrafish homologs of lh1 and lh2 now presents the opportunity for the development of a model system for the in vivo study of these disorders. The zebrafish is an organism proven to be well suited to genetic analysis and the study of embryonic development. In the absence of identified mutations in the zebrafish lh1 or lh2 genes, antisense morpholino oligonucleotides can be used to produce both hypomorphic and null embryonic phenotypes (Nasevicius and Ekker, 2000). Notably, this technique may also be used to distinguish the developmental roles of the lh2 splice variants (Draper et al., 2001). Additionally, both traditional forward genetic screens and reverse genetic approaches (TILLING) may be used to identify fish bearing mutations in lh1 and lh2 (Haffter et al., 1996; Wienholds et al., 2003).
Consistent with their known roles in collagen biosynthesis, our in situ analyses of lh1 and lh2 demonstrate the expression of these genes in tissues shown previously to express various forms of collagen (Sprague et al., 2001). However, the dynamic patterns of lh1 and lh2 expression in a variety of tissues during zebrafish embryogenesis suggest that these genes do not simply play a housekeeping role, but instead participate in specific developmental events. In accordance with this hypothesis, zebrafish lh3, which also displays a restricted pattern of expression, plays a specific role in motor axon migration (Schneider and Granato, 2006). Importantly, our observation that lh1 and lh2 (and lh3) exhibit overlapping and distinct patterns of expression suggests that they likely modify different collagens during development, even though in vitro studies do not reveal strict substrate specificities for these enzymes (Risteli et al., 2004; Schneider and Granato, 2006; Wang et al., 2000). This view is consistent with the observation that the degree to which collagen lysine residues are underhydroxylated in individuals having Ehlers-Danlos syndrome varies with the collagen type and the tissue from which any particular collagen is isolated (Ihme et al., 1984). Comparisons of the patterns of lh gene expression to patterns of collagen expression could reveal which collagens are most likely to be affected in particular tissues in situations of reduced lysyl hydroxylase activity. When coupled with analyses of lysyl hydroxylase deficient embryos, these studies could identify situations in which the different LH enzymes compensate (or fail to compensate) for one another, providing insight into the in vivo differences in LH substrate specificities. In summary, our identification of the zebrafish lysyl hydroxylase genes and their expression patterns now provides the basis for the establishment of a model system that will further understanding of the critical roles these genes play in development and their contribution to disease.
4. Experimental Procedures
4.1 lh1 cloning
The zebrafish LH3 peptide sequence was used to perform a translated BLAST search of the “others” EST database in Genbank in order to identify clones encoding related proteins. ClustalX alignment of translated sequences from identified zebrafish ESTs with multiple vertebrate LH proteins (from zebrafish, rat, mouse and human) demonstrated that several clones with overlapping sequences exhibited strongest homology to LH1 (see also Schneider and Granato, 2006). Complete sequencing of three of these clones revealed that, while two clones, BI896329 and AI958709, contained only partial coding sequences, one of them, AW116639, contained the complete zebrafish lh1 coding sequence, as well as 5′ and ′ UTR sequences.
4.2 lh2 cloning
The zebrafish LH1 and LH3 peptide sequences were used to perform translated BLAST searches of the “others” EST database in Genbank to identify clones encoding zebrafish lh2. A ClustalX alignment of translated sequences from zebrafish clones not encoding lh1 and lh3 with other vertebrate LH proteins identified two overlapping clones, CF265663 and AI585202, whose sequences exhibited homology to the C-termini of LH2 proteins. Additional analysis led to the identification of clone CB365437, whose translated sequence had homology to the N-termini of vertebrate LH2 proteins, but whose nucleotide sequence did not overlap those of CF265663 and AI585202. To identify the intervening zebrafish sequence, the PCR primers 5′ GGGACAGTCAGCCCGTTCTTGAG 3′ (forward) and 5′ TTAGGGATCTACGAAAGACACTGCT 3′(reverse) were used to amplify cDNA prepared from 24 hpf Tü wild-type zebrafish embryos. A PCR product of approximately 2 kb was cloned into pCR-BluntII-TOPO. Sequencing of clones revealed the presence of two transcripts that were identical save for a 63 bp in-frame insertion, corresponding to the two splice variants of lh2 identified in other vertebrate species. These constructs contained the entire lh2 and lh2b coding sequences.
4.3 RT-PCR Analysis
Total mRNA was isolated (Trizol, Invitrogen) from 10 embryos at each of the indicated developmental stages and reverse transcribed to cDNA (Superscript RT II, Invitrogen). lh2 primer pair A: 5′AGTGTGGAACATTCCTTTCCTGGC 3′ (forward), 5′ GAGAAGTGTTGTAGTTGGCAGTGG 3′ (reverse). lh2 primer pair B: 5′AGTGTGGAACATTCCTTTCCTGGC 3′ (forward), 5′ CTTTGGGGATCTGAGCATATCGAATG 3′ (reverse). Actin primers: 5′ AACCCTGCTCACTGAAGCCC 3′ (forward) and 5′ ATGGATGGACCTGCCTCGTC 3′ (reverse).
4.4 In situ Hybridization
Both colorimetric and fluorescent in situ hybridization reactions were performed as described in Schneider and Granato (2006). Colorimetrically labeled embryos were cleared in a 2:1 mixture of benzyl alcohol: benzyl benzoate, mounted in Canada Balsam containing 10% (v/v) methylsalicylate and imaged with a DIC filter on a Zeiss Akioskop microscope. Fluorescently labeled embryos were mounted in Vectashield (Vector Labs) and imaged on a Leica (LCS) confocal microscope.
Acknowledgments
We thank members of the Granato laboratory for helpful discussions and advice on the manuscript. This work was supported by an NRSA postdoctoral fellowship to V.S. and grants from the National Science Foundation and the National Institute of Health to M.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Draper BW, Morcos PA, Kimmel CB. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- Ha-Vinh R, Alanay Y, Bank RA, Campos-Xavier AB, Zankl A, Superti-Furga A, Bonafe L. Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am J Med Genet A. 2004;131:115–120. doi: 10.1002/ajmg.a.30231. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Heikkinen J, Risteli M, Wang C, Latvala J, Rossi M, Valtavaara M, Myllyla R. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. Journal of Biological Chemistry. 2000;275:36158–36163. doi: 10.1074/jbc.M006203200. [DOI] [PubMed] [Google Scholar]
- Ihme A, Krieg T, Nerlich A, Feldmann U, Rauterberg J, Glanville RW, Edel G, Muller PK. Ehlers-Danlos syndrome type VI: collagen type specificity of defective lysyl hydroxylation in various tissues. J Invest Dermatol. 1984;83:161–165. doi: 10.1111/1523-1747.ep12263502. [DOI] [PubMed] [Google Scholar]
- Izsvak Z, Ivics Z, Hackett PB. Repetitive elements and their genetic applications in zebrafish. Biochem Cell Biol. 1997;75:507–523. [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Passoja K, Myllyharju J, Pirskanen A, Kivirikko KI. Identification of arginine-700 as the residue that binds the C-5 carboxyl group of 2-oxoglutarate in human lysyl hydroxylase 1. FEBS Letters. 1998a;434:145–148. doi: 10.1016/s0014-5793(98)00966-1. [DOI] [PubMed] [Google Scholar]
- Passoja K, Rautavuoma K, Ala-Kokko L, Kosonen T, Kivirikko KI. Cloning and characterization of a third human lysyl hydroxylase isoform. Proceedings of the National Academy of Sciences of the United States of America. 1998b;95:10482–10486. doi: 10.1073/pnas.95.18.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirskanen A, Kaimio AM, Myllyla R, Kivirikko KI. Site-directed mutagenesis of human lysyl hydroxylase expressed in insect cells. Identification of histidine residues and an aspartic acid residue critical for catalytic activity. Journal of Biological Chemistry. 1996;271:9398–9402. doi: 10.1074/jbc.271.16.9398. [DOI] [PubMed] [Google Scholar]
- Rautavuoma K, Takaluoma K, Passoja K, Pirskanen A, Kvist AP, Kivirikko KI, Myllyharju J. Characterization of three fragments that constitute the monomers of the human lysyl hydroxylase isoenzymes 1-3. The 30-kDa N-terminal fragment is not required for lysyl hydroxylase activity. Journal of Biological Chemistry. 2002;277:23084–23091. doi: 10.1074/jbc.M112077200. [DOI] [PubMed] [Google Scholar]
- Rautavuoma K, Takaluoma K, Sormunen R, Myllyharju J, Kivirikko KI, Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc Natl Acad Sci U S A. 2004;101:14120–14125. doi: 10.1073/pnas.0404966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli M, Niemitalo O, Lankinen H, Juffer AH, Myllyla R. Characterization of collagenous peptides bound to lysyl hydroxylase isoforms. J Biol Chem. 2004;279:37535–37543. doi: 10.1074/jbc.M405638200. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen H, Sipila L, Kerkela E, Pospiech H, Myllyla R. Characterization of cDNAs for mouse lysyl hydroxylase 1, 2 and 3, their phylogenetic analysis and tissue-specific expression in the mouse. Matrix Biology. 1999;18:325–329. doi: 10.1016/s0945-053x(99)00016-5. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen H, Sipila L, Vapola M, Sormunen R, Salo AM, Uitto L, Mercer DK, Robins SP, Risteli M, Aszodi A, et al. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. J Cell Sci. 2006;119:625–635. doi: 10.1242/jcs.02780. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Granato M. The Myotomal diwanka (lh3) Glycosyltransferase and Type XVIII Collagen Are Critical for Motor Growth Cone Migration. Neuron. 2006;50:683–695. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Sprague J, Doerry E, Douglas S, Westerfield M. The Zebrafish Information Network (ZFIN): a resource for genetic, genomic and developmental research. Nucleic Acids Res. 2001;29:87–90. doi: 10.1093/nar/29.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B, Eyre DR, Shao P. Urinary pyridinoline cross-links in Ehlers-Danlos syndrome type VI. Am J Hum Genet. 1995;57:1505–1508. [PMC free article] [PubMed] [Google Scholar]
- Suokas M, Lampela O, Juffer AH, Myllyla R, Kellokumpu S. Retrieval-independent localization of lysyl hydroxylase in the endoplasmic reticulum via a peptide fold in its iron-binding domain. Biochemical Journal. 2003;370:913–920. doi: 10.1042/BJ20021533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtavaara M, Papponen H, Pirttila AM, Hiltunen K, Helander H, Myllyla R. Cloning and characterization of a novel human lysyl hydroxylase isoform highly expressed in pancreas and muscle. J Biol Chem. 1997;272:6831–6834. doi: 10.1074/jbc.272.11.6831. [DOI] [PubMed] [Google Scholar]
- Valtavaara M, Szpirer C, Szpirer J, Myllyla R. Primary structure, tissue distribution, and chromosomal localization of a novel isoform of lysyl hydroxylase (lysyl hydroxylase 3) J Biol Chem. 1998;273:12881–12886. doi: 10.1074/jbc.273.21.12881. [DOI] [PubMed] [Google Scholar]
- van der Slot AJ, Zuurmond AM, Bardoel AF, Wijmenga C, Pruijs HE, Sillence DO, Brinckmann J, Abraham DJ, Black CM, Verzijl N, et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, Ulrich MM, Middelkoop E, Boers W, Karel Ronday H, DeGroot J, Huizinga TW, Bank RA. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004;23:251–257. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Wang C, Luosujarvi H, Heikkinen J, Risteli M, Uitto L, Myllyla R. The third activity for lysyl hydroxylase 3: galactosylation of hydroxylysyl residues in collagens in vitro. Matrix Biology. 2002;21:559–566. doi: 10.1016/s0945-053x(02)00071-9. [DOI] [PubMed] [Google Scholar]
- Wang C, Valtavaara M, Myllyla R. Lack of collagen type specificity for lysyl hydroxylase isoforms. DNA & Cell Biology. 2000;19:71–77. doi: 10.1089/104454900314582. [DOI] [PubMed] [Google Scholar]
- Wienholds E, van Eeden F, Kosters M, Mudde J, Plasterk RH, Cuppen E. Efficient target-selected mutagenesis in zebrafish. Genome Res. 2003;13:2700–2707. doi: 10.1101/gr.1725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeowell HN, Ha V, Clark WL, Marshall MK, Pinnell SR. Sequence analysis of a cDNA for lysyl hydroxylase isolated from human skin fibroblasts from a normal donor: differences from human placental lysyl hydroxylase cDNA. J Invest Dermatol. 1994;102:382–384. doi: 10.1111/1523-1747.ep12371799. [DOI] [PubMed] [Google Scholar]
- Yeowell HN, Walker LC. Tissue specificity of a new splice form of the human lysyl hydroxylase 2 gene. Matrix Biology. 1999;18:179–187. doi: 10.1016/s0945-053x(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Yeowell HN, Walker LC. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol Genet Metab. 2000;71:212–224. doi: 10.1006/mgme.2000.3076. [DOI] [PubMed] [Google Scholar]