Abstract
Background
In western populations irritable bowel syndrome (IBS) affects between 10% and 30% of the population and has a significant effect on quality of life. It generates a substantial workload in both primary and secondary care and has significant cost implications. Gut-directed hypnotherapy has been demonstrated to alleviate symptoms and improve quality of life but has not been assessed outside of secondary and tertiary referral centres.
Aim
To assess the effectiveness of gut-directed hypnotherapy as a complementary therapy in the management of IBS.
Design of study
Randomised controlled trial.
Setting
Primary care patients aged 18–65 years inclusive, with a diagnosis of IBS of greater than 6 weeks' duration and having failed conventional management, located in South Staffordshire and North Birmingham, UK.
Method
Intervention patients received five sessions of hypnotherapy in addition to their usual management. Control patients received usual management alone. Data regarding symptoms and quality of life were collected at baseline and again 3, 6, and 12 months post-randomisation.
Results
Both groups demonstrated a significant improvement in all symptom dimensions and quality of life over 12 months. At 3 months the intervention group had significantly greater improvements in pain, diarrhoea and overall symptom scores (P<0.05). No significant differences between groups in quality of life were identified. No differences were maintained over time. Intervention patients, however, were significantly less likely to require medication, and the majority described an improvement in their condition.
Conclusions
Gut-directed hypnotherapy benefits patients via symptom reduction and reduced medication usage, although the lack of significant difference between groups beyond 3 months prohibits its general introduction without additional evidence. A large trial incorporating robust economic analysis is, therefore, urgently recommended.
Keywords: hypnotherapy, irritable bowel syndrome, randomised controlled trial
INTRODUCTION
Irritable bowel syndrome (IBS) is a common health problem that affects a substantial proportion of the western population, with point prevalence estimates usually being between 10% and 30%.1–8 The condition generates a substantial workload in both primary and secondary care,7,9 and is a significant cause of morbidity.1 A substantial proportion (17–30%)7,10 of those who present to primary care require referral to a specialist and IBS accounts for 20–50% of referrals to gastroenterology clinics.11–13
People with IBS are more likely than the general population to be too sick to work, have missed days of work and to have visited their doctor.1,5 IBS results in reduced quality of life,1,7,14 adversely affecting patients' general wellbeing, as well as their social, vocational, and sexual functioning. Although outcomes differ to a great extent in terms of health impact and mortality, the effect on quality of life with regards to IBS has been reported to be similar to that observed in congestive heart failure.15 Costs associated with IBS are, therefore, substantial. It has great impact on the individual, industry, and commerce through work absence, as well as the health service.5,6,16
Although many patients do not place ongoing demands on medical services, for some patients, currently available conventional treatment (such as, explanation, reassurance, dietary change, and symptomatic management) is poorly efficacious17,18 and a proportion of them develop chronic disease with high healthcare costs. The majority of individuals with IBS use some form of self-treatment,1 with a proportion trying alternative therapies or management strategies. Gut-directed hypnotherapy has been demonstrated to alleviate symptoms and improve quality of life,19–24 resulting in the establishment of a dedicated NHS tertiary referral centre for the management of refractory IBS. The effectiveness of providing hypnotherapy outside specialised centres or for patients with less severe IBS has not been assessed by means of a randomised controlled trial. This randomised controlled trial aimed to assess the effectiveness of hypnotherapy as a complementary therapy in the primary care management of IBS.
METHOD
Participants
Twenty-four general practices in South Staffordshire and North Birmingham agreed to participate in this study. Eligible patients were defined as those aged 18–65 years, who had been consulting their GP for IBS for more than 6 weeks, and who had failed to manage symptoms using one or more conventional treatments. GPs were requested to manage incident cases according to usual practice and only refer patients if they reconsulted after at least 6 weeks.
Patients who presented with atypical symptoms, such as blood in the stools, where referral was indicated, were excluded. Other exclusions included those who declined consent or were unable to provide informed consent. Patients aged over 65 years were excluded due to the increased risk of serious underlying disease in this older age group.
Those willing to participate were consented by their GP and an appointment for assessment was arranged. All patients were initially seen in a dedicated research clinic by a consultant gastroenterologist, who confirmed eligibility (diagnosis of IBS with no underlying disease) prior to randomisation. Patients for whom additional tests were indicated in order to exclude underlying disease were followed up in the research clinic and only randomised when a diagnosis of IBS was confirmed by the consultant. Although specialist assessment is not a routine feature of primary care IBS management, this step was included in this pilot study to ensure patient safety and to determine the accuracy of primary care diagnoses to inform future work. Patients were, however, recruited from primary care and, therefore, represent this population rather than the selected group of patients referred to secondary care. Baseline data (demographics, symptom score and quality-of-life score) were recorded at the time of randomisation. Patients failing to attend their assessment clinic appointment were offered a second appointment; those not attending two appointments were excluded.
Blocked randomisation was by sealed envelope and was overseen in all clinics by both the consultant gastroenterolgist and one of the research team. Patients were informed of the arm to which they had been randomised, blinding not being possible due to the nature of the intervention. The patient's GP was also informed of the randomisation arm and requested to continue usual management for all participants, as the effectiveness of hypnotherapy was being assessed as a complementary, rather than alternative, therapy.
Intervention
Patients randomised to the intervention (hypnotherapy) were contacted to arrange convenient appointment times. All patients were offered five half-hour sessions of gut-directed hypnotherapy delivered by an experienced hypnotherapist, who had received training in a gut-directed approach. Appointments were approximately 1 week apart and all hypnotherapy sessions took place in a quiet room at Good Hope Hospital in Sutton Coldfield. The initial session involved discussion of symptoms to allow therapy to be appropriately directed as well as an induction of a light state of relaxation/trance.
How this fits in
Gut-directed hypnotherapy has been demonstrated to alleviate symptoms of irritable bowel syndrome and improve quality of life when delivered in specialist centres. This study reports the first attempt to assess the effectiveness of gut-directed hypnotherapy in the primary care management of patients. This piece also adds to the debate around the evaluation of complementary therapies within conventional healthcare settings.
Hypnosis was induced with a standard preliminary hypnotic induction technique (usually an eye-fixation induction technique) followed by standard deepening procedures dependent on the patient's ability. Subsequent sessions strengthened the patient's ability to enter a trance state followed by visualisation techniques, which were determined by the patient's personal symptoms and history. In patients with constipation, for example, this may have included visualisation of the bowel as a river, the current of which gradually speeds up; patients with pain as a primary symptom may have been encouraged to visualise heat in the area of the pain or emanating from a hand placed on the area. All visualisation was patient-directed, with patients being encouraged to use images they felt comfortable with and that represented the symptoms they experienced.
All patients were provided with an autohypnosis tape at the first session and were advised to use this daily, where possible, to induce a state of relaxation in which they should practise the techniques taught to them. The aim of hypnosis in all cases was relaxation and symptom control and no attempts were made at hypnoanalysis.
Control patients received only the research clinic assessment and follow-up questionnaires. Patients in both the intervention and control arm were reminded that their GP retained responsibility for their clinical management and that they should continue to use conventional medication and health services as required and directed.
Outcomes
Primary outcomes comprised an IBS-specific quality-of-life measure25 and a full symptom score, based on the Rome II criteria.1 Self-reported resource utilisation (consultations, as well as prescribed and purchased medications) was also collected for between-group comparison. Outcomes were recorded at baseline and at 3, 6, and 12 months post-randomisation to establish whether treatment had a short-term effect (3-month analysis), and whether benefits were maintained over time.
The symptom score contained three dimensions; pain, constipation, and diarrhoea. The quality-of-life tool had eight dimensions: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual function, and relationships.
Sample size
Previous estimates of variance for quality of life26 indicated that 50 patients in each arm would enable a medium change in scores (standard difference [SD] = 0.6) to be detected with 90% power at the 5% significance level (that is, a change in mean quality of life score from 63 to 77).
Statistical method
Analysis was undertaken on an ‘intention-to-treat’ basis. A repeated measure mixed analysis of variance using all available data (baseline and follow-up) was used to compare the changes in symptoms and quality-of-life scores between the two groups at 3 months post-randomisation and to examine any effects up to 12 months. Treatment alone, together with treatment in combination with time, was considered a fixed effect, with time as a repeated factor and patients as the random factor. χ2 tests were used to compare consultation rates between groups. Little's D test was used to test whether missing data were missing completely at random and multiple imputation methods were used to confirm findings.
RESULTS
Baseline and follow-up
In total, 101 patients were recruited by GPs; 89 of these 101 patients attended the randomisation clinic. From the 89 patients who attended the clinic, 81 met the eligibility criteria and were randomised (Figure 1). Six patients were excluded as their age fell outside of that specified, and two patients were excluded as assessment indicated disease other than IBS (one non-ulcer dyspepsia and one colitis).
Figure 1.
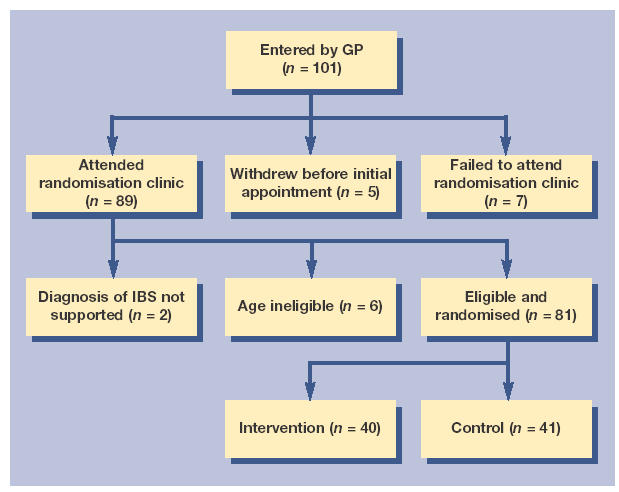
Flowchart of patient inclusion in the study.
The mean age of participating patients was 41.6 years (range 18–65 years) and 69 of the 81 patients were female. Intervention and control groups displayed a similar age distribution (intervention group mean 42.4 years, range 18–65 years; control group mean 40.8 years, range 18–63 years), although slightly more males were included in the intervention than in the control group (8/40 versus 4/41 respectively). Baseline symptom and quality-of-life scores are reported in Tables 1 and 2, respectively, and were generally comparable between the groups although differences were noted in three dimensions of the quality-of-life score: interference with activity (five-unit difference), health worry (five-unit difference), and sexual function (10-unit difference).
Table 1.
Mean change in symptom scores at 3 and 12 months.
| Pain | Constipation | Diarrrhoea | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| C | I | C | I | C | I | C | I | |
| Baseline mean (SE) | 55.0 (3.4) | 52.5 (3.3) | 37.5 (4.2) | 38.4 (4.3) | 34.1 (3.3) | 32.7 (3.1) | 40.4 (2.1) | 39.6 (2.2) |
| Change at 3 months mean (SE) | −6.8 (4.0) | −21.2 (3.4) | −7.1 (2.9) | −9.5 (3.7) | −2.3 (3.4) | −10.2 (2.6) | −4.5 (2.5) | −13.0 (1.8) |
| t (P-valuea) | 2.4 (0.02) | 0.8 (0.41) | 2.0 (0.046) | 2.7 (0.008) | ||||
| Change at 12 months mean (SE) | −15.7 (5.0) | −16.3 (4.1) | 0.8 (4.7) | −3.0 (4.0) | −6.9 (3.6) | −8.4 (3.1) | −6.4 (3.0) | −9.1 (2.6) |
| t (P-valuea) | 0.3 (0.76) | 0.3 (0.74) | 0.7 (0.50) | 0.8 (0.44) | ||||
Repeated measures ANOVA. Negative values for change in scores indicate an improvement in symptoms. Changes in scores were calculated using baseline data from only those cases where follow-up data were available. t statistics refer to comparison of the two arms. C = control. I = intervention. SE = standard error.
Table 2.
Quality-of-life scores at baseline and 3-, 6-, and 12-month follow-up.
| Dysphoria | Interference with activity | Body image | Health worry | Food avoidance | Social reaction | Sexual functioning | Relationships | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | |
| Baseline mean (SE) | 47.5 (4.7) | 47.1 (5.1) | 48.6 (4.4) | 53.8 (4.8) | 51.7 (4.5) | 49.3 (5.3) | 62.2 (3.5) | 57.2 (4.6) | 35.2 (4.8) | 36.2 (5.2) | 49.6 (4.8) | 51.5 (5.0) | 65.2 (5.1) | 55.1 (5.6) | 65.0 (4.5) | 67.3 (4.4) | 51.3 (4.0) | 49.7 (4.5) |
| 3 months mean (SE) | 48.7 (4.7) | 59.9 (5.0) | 46.3 (4.3) | 60.5 (5.3) | 50.6 (4.8) | 58.3 (5.3) | 62.8 (4.4) | 63.4 (4.7) | 35.7 (4.2) | 44.5 (4.9) | 51.2 (4.7) | 58.3 (4.8) | 64.9 (6.0) | 67.8 (5.8) | 67.2 (4.7) | 76.3 (4.5) | 51.5 (3.9) | 60.2 (4.4) |
| 6 months mean (SE) | 51.1 (5.5) | 61.7 (5.6) | 49.2 (4.5) | 61.7 (5.7) | 53.1 (5.5) | 58.8 (5.3) | 62.2 (4.3) | 63.3 (5.3) | 38.0 (5.0) | 42.7 (6.4) | 54.5 (5.3) | 60.7 (5.8) | 69.0 (5.8) | 70.5 (6.1) | 69.5 (5.4) | 79.4 (4.4) | 53.8 (4.5) | 63.3 (5.0) |
| 12 months mean (SE) | 55.1 (6.6) | 68.3 (5.1) | 52.5 (5.4) | 66.3 (5.1) | 50.3 (5.4) | 63.1 (4.9) | 64.2 (5.2) | 69.4 (4.8) | 38.5 (5.4) | 45.3 (5.9) | 54.2 (6.5) | 67.2 (5.4) | 70.7 (6.9) | 74.7 (5.0) | 68.4 (5.7) | 81.4 (4.2) | 55.8 (5.2) | 65.4 (4.4) |
C = control. I = intervention.
Three- and 6-month follow-up was achieved on 82% and 83% of patients, respectively, with no difference between the groups. Twelve-month follow-up was achieved on 65% of patients (29 intervention and 24 control).
Of those patients randomised to the intervention arm, 34 attended all five sessions of hypnotherapy and an additional three patients attended four sessions. Two patients dropped out after only three sessions and one patient failed to attend any therapy sessions.
Symptom scores
Both groups demonstrated a significant improvement in all symptom dimensions over the 12-month period (overall symptoms: t = 4.0, P<0.001). Figure 2 shows the symptom scores (overall, pain, diarrhoea, and constipation) at baseline, 3-, 6-, and 12-month follow-up.
Figure 2.
Mean symptom scores over time.
At 3 months the hypnotherapy group had greater improvements in overall symptom scores (mean change in score from baseline 13.0 versus 4.5, adjusted between group difference 8.5, 95% confidence interval [CI] = 2.3 to 14.7, t = 2.7, P = 0.008) than the control group. There was also weaker evidence for a between-group difference in pain (mean change in score from baseline 21.2 versus 6.8, adjusted between group difference 12.5, 95% CI = 2.4 to 22.6, t = 2.4, P = 0.02), and diarrhoea (10.2 versus 2.3, adjusted between group difference 7.6, 95% CI = 0.2 to 15.1, t = 2.0, P = 0.046) in favour of the intervention group. However, the differences in scores were not maintained over time and at the 12-month follow-up the mean change in score in the intervention group was not significantly superior (Table 1).
Quality of life
Table 2 reports the mean quality-of-life scores at baseline, 3-, 6- and 12-month follow-up. There were significant improvements in scores for both groups (overall quality-of-life score: t = 4.8, P<0.001), as shown in Table 3. However, the mixed modelling analysis, which provides comparisons adjusted for baseline scores, demonstrated no between-group difference in scores at 3 or 12 months (Table 3).
Table 3.
Change in quality-of-life scores over time and mean change in scores at 3 and 12 months.
| Dysphoria | Interference with activity | Body image | Health worry | Food avoidance | Social reaction | Sexual functioning | Relationships | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | |
| Overall change (P-valuea) | 5.6 (<0.001) | 4.2 (<0.001) | 3.0 (0.003) | 2.7 (0.007) | 1.7 (0.08) | 3.1 (0.002) | 3.5 (<0.001) | 3.8 (<0.001) | 4.8 (<0.001) | |||||||||
| Change at 3 months mean (SE) | 5.7 (3.2) | 13.4 (3.3) | 1.9 (2.6) | 9.1 (2.1) | 1.6 (3.3) | 9.0 (3.0) | 3.3 (3.1) | 7.2 (3.2) | −0.4 (4.2) | 10.2 (3.3) | 6.0 (4.5) | 6.6 (3.3) | −0.5 (4.0) | 10.2 (4.7) | 9.2 (3.8) | 8.7 (3.3) | 3.9 (2.6) | 10.1 (2.8) |
| t (P-valuea) | 1.9 (0.06) | 1.8 (0.07) | 1.6 (0.12) | 0.5 (0.61) | 1.5 (0.14) | 0.2 (0.81) | 1.8 (0.07) | 0.3 (0.75) | 1.6 (0.11) | |||||||||
| Change at 12 months mean (SE) | 12.8 (4.6) | 18.6 (3.5) | 8.8 (4.7) | 12.0 (2.8) | 3.7 (5.4) | 9.7 (3.3) | 9.1 (4.3) | 10.1 (3.9) | 9.8 (7.0) | 8.6 (4.6) | 9.4 (4.7) | 10.5 (3.0) | 5.6 (5.2) | 13.5 (6.1) | 11.7 (4.5) | 11.2 (2.7) | 10.9 (4.3) | 13.6 (3.0) |
| t (P-valuea) | 1.2 (0.25) | 1.1 (0.28) | 1.7 (0.09) | 0.7 (0.47) | 0.4 (0.70) | 0.8 (0.41) | 1.6 (0.11) | 0.8 (0.43) | 1.2 (0.24) | |||||||||
Repeated measures ANOVA. Positive values for change in scores indicate an improvement in quality of life. Changes in scores were calculated using baseline data from only those cases where follow-up data were available. C = control, I = intervention.
Other outcomes
Comparison of self-reported GP consultations and self-reported medication use suggested a benefit in the intervention group in terms of reduced medication usage. Some 48% (14/29) of intervention patients reported using prescription medication at some point during the 12-month follow-up period compared with 79% (19/24) of control patients (χ2 = 5.3, P = 0.02). Only 7% (2/29) of intervention patients reported continual use over the 12-month period compared with 42% (10/24) of the control group (χ2 = 9.1, P = 0.003). No difference in the use of over-the-counter (OTC) medication was demonstrated; 13 control patients and 14 intervention patients reported use of OTC medication during the trial period. The range of products purchased for symptom management was wide and included antispasmodics and antidiarrhoeals, ‘natural remedies’ such as probiotics, herbal juices and teas, and other products such as incontinence pads.
None of the control patients reported consulting with a hypnotherapist during the trial period. Three intervention patients and one control patient reported consulting an alternative therapist during the study period: one intervention patient consulted a spiritual healer and two saw a reiki practitioner, while a holistic therapist was consulted by one of the control patients.
DISCUSSION
Summary of main findings
This is the first randomised controlled trial to examine the use of gut-directed hypnotherapy in patients not referred to tertiary care. It is also the first to report long-term outcomes (to 12 months). Both the hypnotherapy and control groups demonstrated some improvement over time. This result is in line with expectations for a trial involving a chronic relapsing condition with patients being recruited at the time of a primary care consultation, which would suggest a relapse in their condition.
Patients randomised to gut-directed hypnotherapy showed significant improvement in symptom scores (pain and diarrhoea) at 3 months compared with control patients, but this between group difference was not sustained over a longer period of time. Other measures used failed to demonstrate any significant between-group differences.
Strengths and limitations of the study
This article does not report a definitive trial of hypnotherapy for IBS and its most notable limitation is that the lack of a third arm (controlling for additional time and attention) means that conclusions can only be drawn about the potential and efficacy of the multicomponent intervention in its entirety. This may or may not be a significant limitation depending on the perspective from which findings are viewed, although identification of active components of a multicomponent therapy is important to minimise both cost and risk of harm.
Incomplete follow-up may have biased symptom and quality-of-life results, however Little's D test indicated that the missing data were missing completely at random (MCAR) (D = 1017, degrees of freedom = 1161, P = 0.99) and statistical analyses using multiple imputation gave similar results to those presented. Our equivocal findings with respect to the benefit of hypnotherapy may be for several reasons. It is possible that hypnotherapy has no significant benefit over conventional treatment alone in terms of symptom and quality-of-life improvement. A second interpretation of the data would be that a benefit does exist, but the study was underpowered to identify this. The study was powered to detect a medium-sized change (SD = 0.6) and aimed to recruit 100 people. In total, 81 were randomised and 53 provided outcomes data at the 12-month follow-up. These 53 patients would enable a medium-sized change to be detected with only 58% power at the 5% significance level. Therefore, it remains a possibility that a type II error has occurred or that a much smaller change in scores is conferred by hypnotherapy; this explanation is indicated by our data in Figure 2.
There also exists the possibility of a type I error due to multiple statistical testing, and noted differences may be spurious, although previous evidence and the consistent trend towards benefit in the intervention arm suggest that differences, where identified, are real. The significant difference in the use of prescription medication suggests that, although conventional measures of symptom and quality of life are not significantly affected by the delivery of hypnotherapy, patients may be able to maintain these scores with reduced recourse to medication. This should be explored further using prescription data. The third interpretation of these data is that a significant benefit is conferred by hypnotherapy but that the outcome measures used were either not sensitive enough to detect this or were inappropriate.
The hypnotherapy group reported a range of benefits suggesting that treatment had improved their ability to manage their symptoms, their confidence, and overall wellbeing. These factors were not routinely measured in this trial and similar data is not available from control patients, meaning attribution bias cannot be excluded. The inclusion of a third control arm and incorporation of more generic outcomes would be advised in future work to allow this to be investigated.
Other explanations relating to the treatment programme may explain our findings. It is possible that gut-directed hypnotherapy does confer long-term benefit for this patient group but the delivery of therapy in this trial was suboptimal and, therefore, benefit not demonstrated. As such, it remains a possibility that delivery of the therapy in another way — for example more sessions, longer sessions, different induction strategies, or a different therapist — may have demonstrated benefit. However, at the outset of this study we aimed to use a therapy programme that, if proving effective, could be accessed and provided by primary care practitioners. It could be argued that longer, intensive programmes would not achieve this aim. In spite of this, future work should aim to evaluate different treatment modes and should evaluate the effect that patient compliance (for example, use of provided audiotapes) has on outcome.
Comparison with existing literature
The finding of a significant difference in overall symptom scores and diarrhoea symptoms between groups mirrors results found in Whorwell et al's original trial,23 where a significantly greater reduction in pain was observed in intervention patients at 12 weeks. That trial did not provide follow-up on both groups beyond 3 months, although long-term follow-up of patients with severe refractory IBS receiving hypnotherapy has been reported elsewhere19 and suggests long-term benefit. Control population data over an extended time period is needed to confirm this, although it is possible that longer-term benefit not demonstrated in this study may be observed in those patients with severe disease as they have greater potential for improvement than patients recruited from primary care.
Implications for future research and clinical practice
Having taken all of these findings into account and put them into context, we report that gut-directed hypnotherapy does benefit patients with IBS in terms of reducing symptoms and medication use. However, the lack of any significant difference between the groups in symptom and quality-of-life scores beyond 3 months would prohibit the more widespread introduction of this therapy without further evidence. The findings of this study support the urgent need for a large well-designed trial incorporating a robust economic analysis.
Acknowledgments
We would like to extend our thanks to the members of the IBS Research Group, in the Department of Primary Care, University of Birmingham. This project has benefited from discussion of the research idea with all members of the group. We are also grateful for the help and support provided by MidReC (Midlands GP Research Consortium) and the practices that collaborated with this study. Thanks also go to the Hynotherapy Unit, University Hospital South Manchester for support during the design of this study and intervention.
Funding body
This study was funded by a research grant from Good Hope R&D (DC/HLR/Singh 2000). Lesley Roberts was in receipt of an NHS R&D New Blood Fellowship (WMidExec 98 Allen) during the period that this work was completed. Sue Wilson is funded by a National Primary Care Career Scientist Award (CSA02/007)
Ethics committee
This study was approved by North Birmingham and South Staffordshire Local Research Ethics Committees (Reference 549.00 and TM/SJH/Wilson Hypnotherapy for IBS, respectively)
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Wilson S, Roberts L, Roalfe A, et al. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract. 2004;54:495–502. [PMC free article] [PubMed] [Google Scholar]
- 2.Agreus L, Svardsudd K, Nyren O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109(3):671–680. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WG, Heaton KW. Functional bowel disorders in apparently healthy people. Gastroenterology. 1980;79(2):283–288. [PubMed] [Google Scholar]
- 4.Kennedy TM, Jones RH, Hungin AP, et al. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut. 1998;43(6):770–774. doi: 10.1136/gut.43.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 6.Talley NJ, Gabriel SE, Harmsen WS, et al. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109(6):1736–1741. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 7.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40 000 subjects. Aliment Pharmacol Ther. 2003;17(5):643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones R, Lydeard S. Irritable bowel syndrome in the general population. BMJ. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46(1):78–82. doi: 10.1136/gut.46.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drossman D, Corazziari E, Talley N, Thompson W, Whitehead W. Rome II: The functional gastrointestinal disorders: diagnosis, pathophysiology and treatment: a multinational consensus. McLean: Degnon Associates; 2000. [Google Scholar]
- 11.Harvey RF, Salih SY, Read AE. Organic and functional disorders in 2000 gastroenterology outpatients. Lancet. 1983;1:632–634. doi: 10.1016/s0140-6736(83)91802-0. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson A, Sircus W, Eastwood MA. Frequency of ‘functional’ gastrointestinal disorders. Lancet. 1977;2:613–614. doi: 10.1016/s0140-6736(77)91466-0. [DOI] [PubMed] [Google Scholar]
- 13.Fielding JF. A year in out-patients with the irritable bowel syndrome. Ir J Med Sci. 1977;146(6):162–166. doi: 10.1007/BF03030953. [DOI] [PubMed] [Google Scholar]
- 14.O'Keefe EA, Talley NJ, Zinsmeister AR, Jacobsen SJ. Bowel disorders impair functional status and quality of life in the elderly: a population-based study. J Gerontol A Biol Sci Med Sci. 1995;50(4):M184–M189. doi: 10.1093/gerona/50a.4.m184. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead WE, Burnett CK, Cook EW, III, Taub E. Impact of irritable bowel syndrome on quality of life. Dig Dis Sci. 1996;41(11):2248–2253. doi: 10.1007/BF02071408. [DOI] [PubMed] [Google Scholar]
- 16.Everhart JE, Renault PF. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology. 1991;100(4):998–1005. doi: 10.1016/0016-5085(91)90275-p. [DOI] [PubMed] [Google Scholar]
- 17.Francis CY, Whorwell PJ. Bran and irritable bowel syndrome: time for reappraisal. Lancet. 1994;344:39–40. doi: 10.1016/s0140-6736(94)91055-3. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, Naveau S, Mory B, Chaput JC. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8(5):499–510. doi: 10.1111/j.1365-2036.1994.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 19.Gonsalkorale WM, Miller V, Afzal A, Whorwell PJ. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut. 2003;52(11):1623–1629. doi: 10.1136/gut.52.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonsalkorale WM, Houghton LA, Whorwell PJ. Hypnotherapy in irritable bowel syndrome: a large-scale audit of a clinical service with examination of factors influencing responsiveness. Am J Gastroenterol. 2002;97(4):954–961. doi: 10.1111/j.1572-0241.2002.05615.x. [DOI] [PubMed] [Google Scholar]
- 21.Houghton LA, Heyman DJ, Whorwell PJ. Symptomatology, quality of life and economic features of irritable bowel syndrome — the effect of hypnotherapy. Aliment Pharmacol Ther. 1996;10(1):91–95. doi: 10.1111/j.1365-2036.1996.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 22.Whorwell PJ, Prior A, Colgan SM. Hypnotherapy in severe irritable bowel syndrome: further experience. Gut. 1987;28(4):423–425. doi: 10.1136/gut.28.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet. 1984;2:1232–1234. doi: 10.1016/s0140-6736(84)92793-4. [DOI] [PubMed] [Google Scholar]
- 24.Harvey RF, Hinton RA, Gunary RM, Barry RE. Individual and group hypnotherapy in treatment of refractory irritable bowel syndrome. Lancet. 1989;1:424–425. doi: 10.1016/s0140-6736(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 25.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 26.Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95(4):999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]



