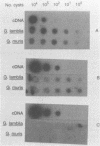
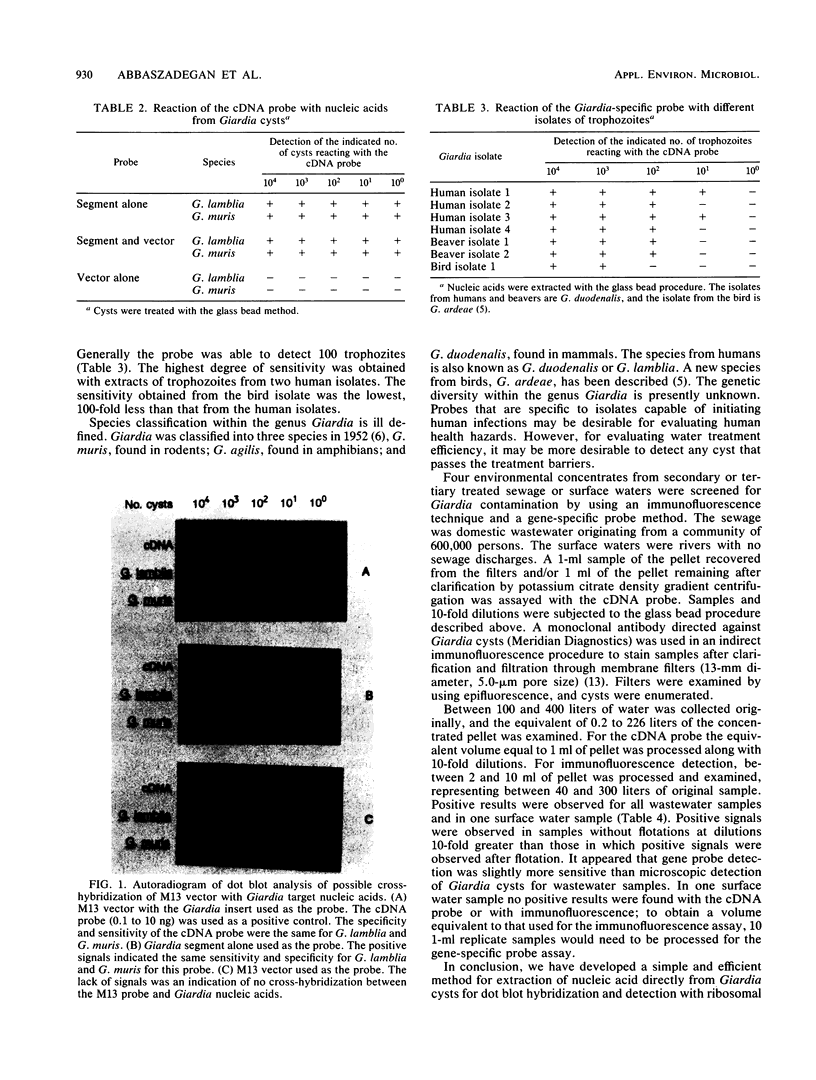
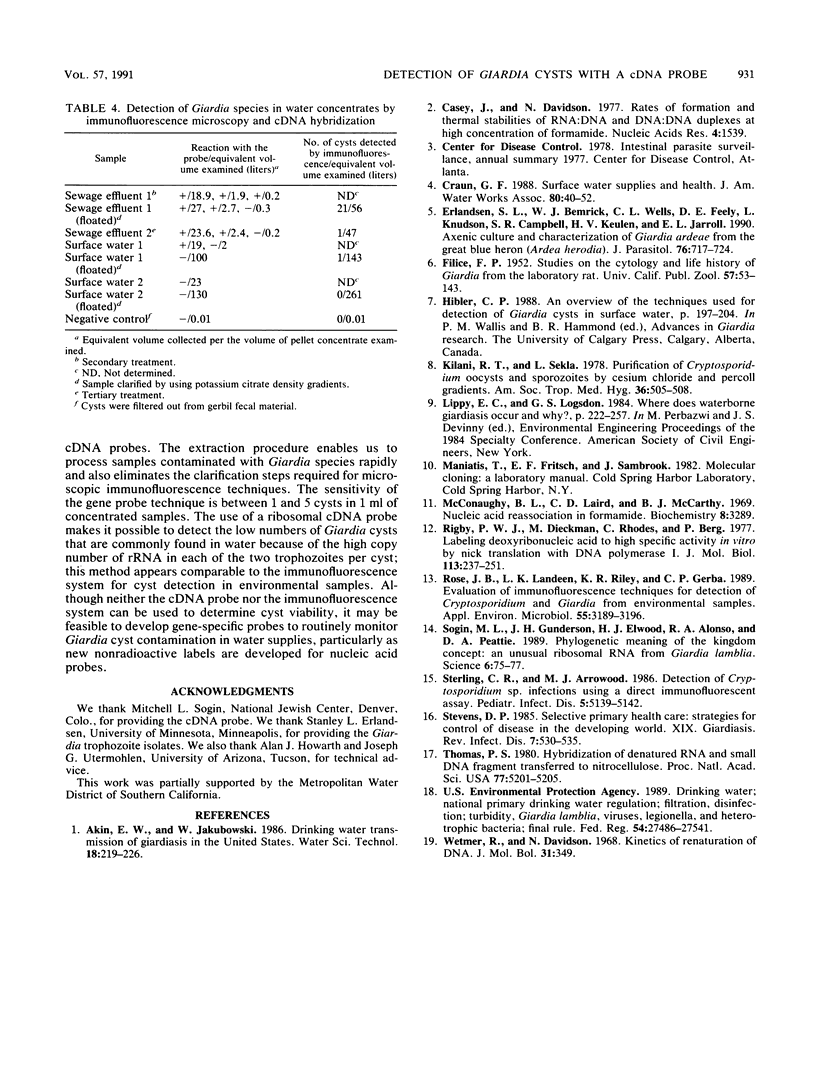
Abstract
Giardiasis is the most common human parasite infection in the United States, causing a lengthy course of diarrhea. Transmission of Giardia species is by the fecal-oral route, and numerous waterborne outbreaks have been documented. The Environmental Protection Agency has regulated Giardia species in drinking water through the Surface Water Treatment Rule. Current methods for detection of Giardia species in water rely primarily on microscopic observation of water concentrates with immunofluorescence techniques. We evaluated the efficacy of using a gene-specific probe for the detection of Giardia species in water. A cDNA probe, 265 bp long, from the small subunit of rRNA of Giardia lamblia was used for detection of cysts. The replicative form of the M13 vector with an insert was isolated from lysed host Escherichia coli XL1-Blue and used for production of the cDNA probe by nick translation with 32P-labeled nucleotides. Six different protocols were tested for extracting nucleic acids from the cysts. With the most efficient procedure, disrupting Giardia cysts with glass beads in the presence of proteinase K, as few as 1 to 5 cysts per ml can be detected in water sample concentrates with dot blot hybridization assays.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Bemrick W. J., Wells C. L., Feely D. E., Knudson L., Campbell S. R., van Keulen H., Jarroll E. L. Axenic culture and characterization of Giardia ardeae from the great blue heron (Ardea herodias). J Parasitol. 1990 Oct;76(5):717–724. [PubMed] [Google Scholar]
- Kilani R. T., Sekla L. Purification of Cryptosporidium oocysts and sporozoites by cesium chloride and Percoll gradients. Am J Trop Med Hyg. 1987 May;36(3):505–508. doi: 10.4269/ajtmh.1987.36.505. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose J. B., Landeen L. K., Riley K. R., Gerba C. P. Evaluation of immunofluorescence techniques for detection of Cryptosporidium oocysts and Giardia cysts from environmental samples. Appl Environ Microbiol. 1989 Dec;55(12):3189–3196. doi: 10.1128/aem.55.12.3189-3196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Stevens D. P. Selective primary health care: strategies for control of disease in the developing world. XIX. Giardiasis. Rev Infect Dis. 1985 Jul-Aug;7(4):530–535. doi: 10.1093/clinids/7.4.530. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]