Abstract
Background
Opportunistic screening for genital chlamydia infection is being introduced in England, but evidence for the effectiveness of this approach is lacking. There are insufficient data about young peoples' use of primary care services to determine the potential coverage of opportunistic screening in comparison with a systematic population-based approach.
Aim
To estimate use of primary care services by young men and women; to compare potential coverage of opportunistic chlamydia screening with a systematic postal approach.
Design of study
Population based cross-sectional study.
Setting
Twenty-seven general practices around Bristol and Birmingham.
Method
A random sample of patients aged 16–24 years were posted a chlamydia screening pack. We collected details of face-to-face consultations from general practice records. Survival and person-time methods were used to estimate the cumulative probability of attending general practice in 1 year and the coverage achieved by opportunistic and systematic postal chlamydia screening.
Results
Of 12 973 eligible patients, an estimated 60.4% (95% confidence interval [CI] = 58.3 to 62.5%) of men and 75.3% (73.7 to 76.9%) of women aged 16–24 years attended their practice at least once in a 1-year period. During this period, an estimated 21.3% of patients would not attend their general practice but would be reached by postal screening, 9.2% would not receive a postal invitation but would attend their practice, and 11.8% would be missed by both methods.
Conclusions
Opportunistic and population-based approaches to chlamydia screening would both fail to contact a substantial minority of the target group, if used alone. A pragmatic approach combining both strategies might achieve higher coverage.
Keywords: chlamydia trachomatis, family practice, mass screening, primary health care
INTRODUCTION
A National Chlamydia Screening Programme is being phased in throughout England to reduce the transmission of and morbidity associated with genital Chlamydia trachomatis infection.1 The screening programme is based on opportunistic screening of men and women under 25 years attending selected healthcare settings.1 The programme pilot only targeted women,2,3 so research is needed to determine the settings in which men would be most efficiently covered by opportunistic screening. Primary care is likely to be a key location since nearly half the cases of infection in women in the pilot studies were identified in general practices.2
Systematic screening, in which all individuals in the target group are invited by post to take part, is an alternative to opportunistic testing. The NHS cervical and breast cancer prevention programmes, which use this approach, rely on GPs' lists of patients' postal addresses. Accuracy is therefore essential for high screening coverage. GPs' lists are known to include patients who no longer live at their registered address, but up-to-date estimates in young adults are not available, and little is known about the consulting behaviour of these patients.4–6
How this fits in
Opportunistic screening for genital chlamydia in young adults is being implemented in selected settings in England, although evidence of effectiveness from randomised trials is lacking. There are insufficient data about young people's use of primary care services to determine whether the coverage achieved by opportunistic chlamydia screening would be greater than that of a systematic population approach. The majority (60%) of men aged 16–24 years were estimated to have visited their general practice each year: this is more often than is assumed. A chlamydia screening strategy combining systematic and opportunistic elements would have the highest coverage.
Young men are presumed not to be reachable by opportunistic interventions in primary care because they have the lowest consultation rates of any age group: 1.7 consultations per male aged 20–24 years compared with women of the same age, who are the most frequent users (5.0 per year).7 These averages include telephone calls and multiple consultations, and cannot therefore be used to estimate the proportion of a practice population that would be covered by a primary care-based intervention.
The Chlamydia Screening Studies project (ClaSS) included an assessment of systematic postal screening for chlamydia using home-collected and mailed specimens from young men and women.8 We used this opportunity to investigate the use of primary care services by men and women aged 16–24 years in the same population, and could thus compare the potential coverage of opportunistic and postal chlamydia screening strategies.
METHOD
Details of the ClaSS project have previously been described.8,9 Briefly, we recruited 27 general practices from research networks in the Bristol and West Midlands areas, to include urban, suburban and rural areas, and populations with different levels of material deprivation and ethnic groups. The distribution of patients by age, sex, and ethnic group was broadly similar to that of England and Wales as a whole. We randomly selected a fixed proportion of men and women aged 16–39 years in each practice.
Home-based postal chlamydia screening
Selected patients were invited by post to collect a first-void urine sample at home, and women were also asked for a self-administered vulvo-vaginal swab. Participants mailed their samples in prepaid envelopes to their local Public Health Laboratory Service (now Health Protection Agency) laboratory. Study packs were sent out between February 2001 and July 2002 using the contact addresses held by general practices. Packs were sent by either recorded delivery (first four practices) or courier (23 practices). Couriers made up to five attempts to deliver packs, including at least one visit after 6 pm or at a weekend, and research staff made the same number of visits to non-responders whose packs had been delivered by mail. We also sent reminder letters and made up to three telephone calls to people who did not return a specimen. A random 5% sample of households contacted by couriers were recontacted by research staff to check the data for accuracy. We classified individuals as ‘participants’ (returned a postal specimen), ‘refusers’ (responded to indicate they did not wish to participate), ‘non-responders’ (known to have received a pack but did not respond) or ‘ghosts’ (confirmed as not resident at the address held by the practice or not contactable by any method).
Consultation patterns in primary care
Between June and August 2002 we obtained details of doctor–patient or nurse–patient contacts for all selected patients. Practice staff provided anonymised details of whether the patient was still registered with the practice and the date of their most recent attendance. Data collection in each practice took place after all packs had been delivered. Those collecting data were unaware of whether or not patients had participated in the study.
Statistical analysis
Analysis was restricted to 16–24 year olds. We used survival analysis to take into account the variable follow-up period and the fact that some individuals changed practices during the study period. For each individual invited to participate in the screening study, we measured time at risk of consulting from the date his or her study pack was sent out. We censored observations at the date of the last attendance, or at the date of data collection, whichever was first. For individuals no longer registered with the practice who had no recorded attendance, we did not know the date that they had left, so we censored these observations at the midpoint between the pack being sent out and the date of data collection.
We used Kaplan–Meier methods to estimate the cumulative probability (with 95% confidence interval [CI]) of patients aged 16–24 years consulting the practice at least once within 1 year of the study pack being sent out, and stratified estimates according to age group, sex, chlamydia test result, and whether they could be contacted by post. We used the cumulative probabilities of consulting stratified by whether patients could be contacted by post or not, to estimate the proportions of patients aged 16–24 years who would be covered by opportunistic and systematic screening strategies over a 1-year period.
All statistical analyses were conducted using Stata 8.2 (Stata Corporation, Austin, TX, US).
RESULTS
Of 19 773 patients invited to participate in the ClaSS project screening study, 15 319 were aged 16–24 years. We excluded data from 2084 (13.6%) patients from three practices that provided no data, and 262 (1.7%) records with implausible dates. We therefore analysed data from 12 973 (84.7%) patients aged 16–24 years in 24 practices. The distribution of patients by age and sex was similar for those with and without complete data. Among patients with complete data, 2705 (20.9%) were found to be ‘ghosts’. Of the remaining 10 268 patients, 3318 (32.3%) returned a specimen, 1364 (13.3%) declined to participate, and 5586 (54.4%) did not respond in any way.
Table 1 shows the estimated cumulative probabilities of men and women aged 16–24 years consulting their general practice in the year after they were invited to participate in postal screening. Overall, an estimated 68.6% (95% CI = 67.3 to 69.9%) of patients had consulted their practice by 12 months, and 89.3% (95% CI = 88.1 to 90.3%) by 17.5 months, the maximum follow-up period. The probability of consulting was higher in women (75%) than men (60%, P<0.0001), and in those who could be contacted at their registered address (73%) than ‘ghost’ patients (44%, P<0.0001). When stratified by sex, the probability of men consulting was higher in 16–19 (64%) than 20–24 year olds (58%, P = 0.004), and among those participating in postal screening, slightly higher in those with positive (72%) compared with negative chlamydia test results (69%, P = 0.036). Consultation patterns in women did not vary by age or chlamydia test result.
Table 1.
Cumulative probability of consulting general practice in 1 year, men and women aged 16–24 years.
| Men | Women | Men and women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Consulted, total | % Consulting by 1 year (95% CI) | P value | Consulted, total | % Consulting by 1 year (95% CI) | P value | Consulted, total | % Consulting by 1 year (95% CI) | P value |
| All | 2152 | 60.4 (58.3 to 62.5) | 3435 | 75.3 (73.7 to 76.9) | 5587 | 68.6 (67.3 to 69.9) | |||
| Age group | 0.196 | ||||||||
| 16–19 years | 975 | 64.0 (60.9 to 67.1) | 0.004 | 1441 | 75.5 (73.1 to 77.9) | 0.340 | 2416 | 70.3 (68.3 to 72.3) | |
| 20–24 years | 1177 | 57.5 (54.7 to 60.4) | 1994 | 75.0 (72.8 to 77.2) | 3171 | 67.2 (65.4 to 69.0) | |||
| Participation status | <0.0001 | <0.0001 | <0.0001 | ||||||
| Responded | 597 | 69.1 (65.0 to 73.2) | 1238 | 79.9 (77.4 to 82.3) | 1835 | 75.9 (73.7 to 78.1) | |||
| Refused | 238 | 50.6 (45.2 to 56.3) | 404 | 65.5 (60.8 to 70.1) | 642 | 58.8 (55.2 to 62.5) | |||
| Did not respond | 1052 | 69.2 (66.1 to 72.3) | 1401 | 81.9 (79.4 to 84.2) | 2453 | 75.7 (73.7 to 77.7) | |||
| Ghost | 265 | 33.7 (29.3 to 38.5) | 392 | 55.3 (49.9 to 60.9) | 657 | 44.1 (40.5 to 47.8) | |||
| Contactabilitya | <0.0001 | ||||||||
| Contactable | 1887 | 65.9 (63.7 to 68.2) | <0.0001 | 3043 | 78.4 (76.7 to 80.0) | <0.0001 | 4930 | 72.9 (71.6 to 74.3) | |
| Not contactable | 265 | 33.7 (29.3 to 38.5) | 392 | 55.3 (49.9 to 60.9) | 657 | 44.1 (40.5 to 47.8) | |||
| Chlamydia resultb | 0.036 | 0.969 | 0.154 | ||||||
| Positive | 39 | 71.7 (56.7 to 85.1) | 76 | 73.8 (62.9 to 83.7) | 115 | 73.3 (64.5 to 81.4) | |||
| Negative | 557 | 69.1 (64.8 to 73.3) | 1161 | 80.4 (77.8 to 82.8) | 1718 | 76.2 (73.9 to 78.4) | |||
Patients who could be contacted included everyone except those classified as ‘ghost’ patients.
Based on a subsample of 3318 responders who provided a specimen.
Figure 1 illustrates the estimated proportion of people who would potentially be contacted in 1 year of an opportunistic or a systematic chlamydia screening programme. Slightly more than half (57.7%) would be contacted by either strategy. However, 21.3% of people would not attend the practice but would receive an invitation at their home address, whereas 9.2% of people would not have received a postal invitation, but would attend their surgery. The remaining 11.8% of people would not be reached by either strategy. These figures do not take into account the proportions attending primary care who would actually be tested, or whether a specimen was actually returned.
Figure 1.
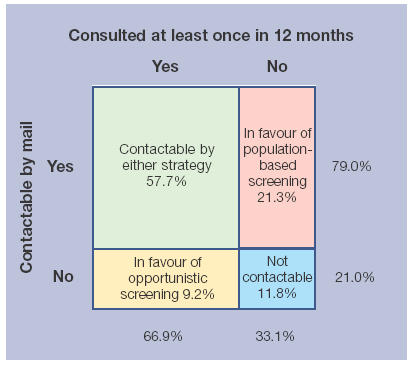
The potential coverage achieved by opportunistic and population-based screening.
DISCUSSION
Summary of main findings
In this study, the majority of both men and women aged 16–24 years attended their general practice at least once in a 1-year period. Four-fifths of patients received an invitation to participate in postal chlamydia screening. An estimated 21% of patients would not be reached by opportunistic screening in primary care but would receive a postal invitation.
Comparison with existing literature
The standard method of calculating and presenting consultation rates demonstrates relative differences by age and sex but does not show who does or does not attend the practice.7,10 It was estimated in 1991–1992 that 78% of all patients attend their practice each year, but proportions stratified by age and sex were not provided.10 We applied survival methods to estimate primary care use in a way that is more useful to those planning interventions: the cumulative proportion of patients attending the practice in a 1-year period. This showed that that the majority of both men and women aged 16–24 years attended their practice at least once during the 1-year study period.
About one-fifth of young men and women were not contactable at the address registered with their general practice, but 44% of them continued to use that practice as their source of primary medical care. This suggests that many of these ‘ghost’ patients are temporarily away from home or have moved locally but have not informed their practice of their new address. Our estimate of the proportion of ‘ghost’ patients in 16–24 year olds is consistent with those in older age groups, reflecting both the inaccuracies of general practice registers and patient mobility.4–6 This study highlights the need to improve the accuracy of registers maintained by general practices, which also constitute the central patient register used by the NHS cervical and breast cancer screening programmes. Young adults might not see the need to inform their practice of frequent changes of address, so practices should use all contacts as an opportunity to keep records up to date.
Strengths and limitations of the study
The strengths of this study are that: we analysed data from nearly 13 000 men and women from 24 diverse general practices in two large geographic areas, so our results should be generalisable to the UK; we took extensive measures to verify the registration and residential status of all potential participants; and we examined the potential coverage of both opportunistic and systematic chlamydia screening in the same population.
Our study has some limitations. First, we might have overestimated attendance during the study period due to attendances by patients with positive test results and non-specific effects of the intervention itself. We think that these factors would overestimate the probability of consulting by less than 10% for the following reasons: our 1-year estimate for women aged 16–24 years (75%) is consistent with the 69% of women of the same age in the Portsmouth chlamydia screening pilot study who attended their general practice in 1 year;3 and patients with positive chlamydia results had a similar probability of consulting to those with negative results (Table 1). Second, despite extensive fieldwork, we might still have misclassified some individuals and overestimated the proportion of ‘ghosts’. Third, data about consultations were anonymised to comply with data protection regulations, so we could not check the accuracy of these data or complete missing observations. Fourth, to simplify data collection for practices we only recorded the most recent consultation so we could not analyse multiple attendances.
Implications for general practice and future research
The presumed poor use of primary care by young men was one reason why men were originally not to be included in a chlamydia screening programme.11 Instead men would be captured in genitourinary clinics either due to symptomatic infection, or through contact tracing. Although the National Chlamydia Screening Programme encourages the involvement of men, of 17 000 samples received in the first year of screening in 10 locations, only 10% came from primary care and only 10% were from men.1 Our findings strengthen the arguments for offering chlamydia screening to men in primary care: prevalence is as high in men as in women,12,13 partner notification is often not done,14,15 and men's perceived responsibility for sexual health would increase.16,17 In fact, the under-representation of men in opportunistic chlamydia screening in Sweden is thought to be one reason why chlamydia transmission has not been controlled.18
Randomised trials have shown that systematic chlamydia screening can reduce the incidence of pelvic inflammatory disease, but these results cannot necessarily be extrapolated to opportunistic screening, for which trials of effectiveness have not been conducted.18 The effectiveness of both opportunistic and systematic screening approaches depends in part on coverage (whether people receive an offer of testing) and also the uptake of the offer of screening. The effective screening rate for opportunistic programmes depends on the proportion of those consulting who are offered and accept a test.3 In the national chlamydia screening pilots, the proportion of women offered a test in primary care was not recorded, but an estimated 46% of sexually active women under 25 years in the Portsmouth site were tested, and 9% had chlamydia.3 The high screening rate in general practice was probably assisted by substantial financial incentives, which will not be available in the National Chlamydia Screening Programme. This is one of several obstacles to introducing opportunistic chlamydia screening in practice in primary care.19
Systematic home-based testing can provide chlamydia screening for people who do not use health services, or who are not offered opportunistic tests. In the main ClaSS project the uptake of systematic postal screening (35%) was low, but similar to that in comparable studies of home-based screening, and of opportunistic screening studies where incentives were not offered.3,12,20–22 The low response rate might have been partly due to the complexity of the research project and lack of familiarity with home sampling. Widespread publicity would probably increase participation if systematic screening were introduced as part of a national programme.
Opportunistic and systematic screening approaches are not mutually exclusive. In practice, the cervical screening and childhood immunisation programmes both use general practice registers as the basis for systematic screening, and offer smear tests or vaccination opportunistically to those missed by postal invitation. A combined approach to chlamydia screening should also be considered. The English National Chlamydia Screening Programme is currently based on opportunistic testing, but coverage could be increased by sending periodic invitations to young adult men and women who have not attended the practice recently, inviting them to either mail a home-collected specimen or attend their general practice. Further research would be required to establish the screening interval. To optimise the effectiveness of chlamydia screening, further research about the acceptability to patients, and the population impact and cost-effectiveness of alternative strategies is also required. The ClaSS project will provide important information about these issues.
In summary, by calculating a new measure of primary care use we showed that young men consult more frequently than previously believed. Empirical data about the potential coverage of opportunistic and systematic chlamydia screening approaches suggest that a combined approach might achieve higher coverage than either strategy alone.
Acknowledgments
The members of the ClaSS project group are: Matthias Egger, Rona Campbell, Owen Caul, George Davey Smith, Anna Graham, Alan Herring, Richard Hobbs, Paddy Horner, Mia Huengsberg, Nicola Low, John Macleod, Anne McCarthy, Tracy Roberts, Jonathan Ross, Chris Salisbury, Susan Skidmore, and Jonathan Sterne. We would also like to thank Anna Graham for her comments on this paper and the staff and patients of the participating general practices for their help.
Funding body
ClaSS is funded through the NHS R&D HTA programme, project number 97/32/31. This research has been conducted by the authors independently of the funding body. John Macleod and Nicola Low are supported by NHS Career Scientist awards
Ethics committee
The study was approved by the South West Multicentre Research Ethics Committee (MREC/00/6/30)
Competing interests
The authors have stated that there are none
REFERENCES
- 1.LaMontagne DS, Fenton KA, Randall S, et al. on behalf of the National Chlamydia Screening Steering Group Establishing the National Chlamydia Screening Programme in England: results from the first full year of screening. Sex Transm Infect. 2004;80:335–341. doi: 10.1136/sti.2004.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimenta JM, Catchpole M, Rogers PA, et al. Opportunistic screening for genital chlamydial infection. II: prevalence among healthcare attenders, outcome, and evaluation of positive cases. Sex Transm Infect. 2003;79:22–27. doi: 10.1136/sti.79.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimenta JM, Catchpole M, Rogers PA, et al. Opportunistic screening for genital chlamydial infection. I: acceptability of urine testing in primary and secondary healthcare settings. Sex Transm Infect. 2003;79:16–21. doi: 10.1136/sti.79.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowling A, Jacobson B. Screening: the inadequacy of population registers. BMJ. 1989;298:545–546. doi: 10.1136/bmj.298.6673.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robson J, Falshaw M. Audit of preventive activities in 16 inner London practices using a validated measure of patient population, the ‘active patient’ denominator. Healthy Eastenders Project. Br J Gen Pract. 1995;45:463–466. [PMC free article] [PubMed] [Google Scholar]
- 6.Macleod J, Rowsell R, Horner P, et al. Postal urine specimens: Are they a feasible method for genital chlamydial infection screening? Br J Gen Pract. 1999;49:455–458. [PMC free article] [PubMed] [Google Scholar]
- 7.Rowlands S, Moser K. Consultation rates from the general practice research database. Br J Gen Pract. 2002;52:658–660. [PMC free article] [PubMed] [Google Scholar]
- 8.Low N, McCarthy A, Macleod J, et al. The chlamydia screening studies: rationale and design. Sex Transm Infect. 2004;80:342–348. doi: 10.1136/sti.2003.006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chlamydia screening studies. www.chlamydia.ac.uk (accessed 13 Dec 2005)
- 10.McCormick A, Fleming D, Charlton J. MB5 Office of Population Censuses and Surveys. Morbidity Statistics from General Practice. Fourth national study 1991–1992. No. 3. London: HMSO; 2004. [Google Scholar]
- 11.CMO's Expert Advisory Group. Chlamydia trachomatis. London: Department of Health; 1998. [Google Scholar]
- 12.Macleod J, Salisbury C, ClaSS Study Group Prevalence of genital Chlamydia trachomatis infection in an UK general population sample. Sex Transm Infect. 2003;79:A1. [Google Scholar]
- 13.Fenton KA, Korovessis C, Johnson AM, et al. Sexual behaviour in Britain: reported sexually transmitted infections and prevalent genital Chlamydia trachomatis infection. Lancet. 2001;358:1851–1854. doi: 10.1016/S0140-6736(01)06886-6. [DOI] [PubMed] [Google Scholar]
- 14.Low N, Huengsberg M, ClaSS Study Group Can partner notification be done in primary care? Randomised controlled trial. Sex Transm Infect. 2003;79:A6. [Google Scholar]
- 15.Gustafsson B, Parment PA, Ramstedt K, Wikstrom A. [A questionnaire study in the county of Stockholm on transmission control of chlamydia infections. Too many physicians neglect the contact tracing]. [Swedish] Lakartidningen. 2000;97:3269–3272. [PubMed] [Google Scholar]
- 16.Duncan B, Hart G. Sexuality and health: the hidden costs of screening for Chlamydia trachomatis. BMJ. 1999;318:931–933. doi: 10.1136/bmj.318.7188.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton KA. Screening men for Chlamydia trachomatis infection: have we fully explored the possibilities? Commun Dis Public Health. 2000;3:86–89. [PubMed] [Google Scholar]
- 18.Low N, Egger M. What should we do about screening for genital chlamydia? Int J Epidemiol. 2002;31:891–893. doi: 10.1093/ije/31.5.891. [DOI] [PubMed] [Google Scholar]
- 19.McNulty CA, Freeman E, Bowen J, et al. Barriers to opportunistic chlamydia testing in primary care. Br J Gen Pract. 2004;54:508–514. [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen B, Olesen F, Moller JK, Ostergaard L. Population-based strategies for outreach screening of urogenital Chlamydia trachomatis infections: a randomized, controlled trial. J Infect Dis. 2002;185:252–258. doi: 10.1086/338268. [DOI] [PubMed] [Google Scholar]
- 21.Grun L, Tassano-Smith J, Carder C, et al. Comparison of two methods of screening for genital chlamydial infection in women attending in general practice: cross sectional survey. BMJ. 1997;315:226–230. doi: 10.1136/bmj.315.7102.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobin C, Aggarwal R, Clarke J, et al. Chlamydia trachomatis: opportunistic screening in primary care. Br J Gen Pract. 2001;51:565–566. [PMC free article] [PubMed] [Google Scholar]


