Abstract
Background
There are limited data about the longer-term outcomes in acute otitis media (AOM) when comparing the realistic alternatives of immediate prescription of antibiotics and a ‘wait and see’ or delayed prescribing policy.
Aim
The aim was to assess the medium and longer term outcomes of two prescribing strategies for otitis media.
Design of study
Follow-up of a randomised controlled trial cohort.
Setting
Primary care.
Method
Three-hundred and fifteen children aged 6 months to 10 years presenting with AOM were randomised to immediate antibiotics, or antibiotics delayed at the parents discretion 72 hours if the child still had significant otalgia or fever, or was not improving. Episodes of earache since study entry were documented, and a poor score (of 9 or more — the top 20%) on a reliable six-item functional rating scale (Cronbach's α = 0.75).
Results
The delayed prescribing strategy did not significantly increase reported episodes of earache in the 3 months since randomisation (odds ratio [OR] = 0.89; 95% confidence interval [CI] = 0.48 to 1.65) or over 1 year (OR = 1.03; 95% CI = 0.60 to 1.78) nor of poor scores on the function scale at 3 months (OR = 1.16; 95% CI = 0.61 to 2.22) or 1 year (OR = 1.12; 95% CI = 0.57 to 2.19), and controlling for subsequent antibiotic use after the randomised episode did not alter these estimates. The number of prior episodes of AOM documented in the doctor's notes predicted episodes of earache reported (0, 1, ≥2 episodes, respectively; OR = 1, 2.42, 2.61; χ2 for trend 8.04; P<0.01). There was weaker evidence that prior episodes also predicted poor function at 1 year (OR = 1, 1.86, 2.28; χ2 for trend 5.49; P = 0.019). For children with recurrent AOM (two or more previous episodes documented in the doctor's notes, n = 43) there was possible evidence of fewer episodes of earache in the 3 months since study entry in the immediate antibiotic group (10% compared to 39% in the delayed group, χ2 4.8, P = 0.029), but no effect from randomisation to 1 year.
Conclusions
For most children, delayed prescribing is not likely to have adverse longer-term consequences. Children with recurrent AOM are more likely to have poorer outcomes. Secondary analysis should be treated with caution and requires confirmation, but suggests that treating such children with antibiotics immediately may not alter longer-term outcomes.
Keywords: antibiotics, otitis media, prescribing strategies, randomised controlled trial
INTRODUCTION
Otitis media is one of the most common acute respiratory conditions managed in primary care, yet treatment is controversial.1–3 Evidence from systematic reviews suggests marginal benefit from antibiotics for most children:4 an estimated 18 children have to be treated for one child to benefit from symptom resolution during the next week. Prescribing for all children is also likely to encourage attendance in future episodes, increase pressure on doctors to prescribe, increase antibiotic use,5–8 and increase antibiotic resistance.3,9 A large Dutch cohort documented that waiting for 72 hours before symptomatic treatment is safe: the only child to develop mastoiditis was not given antibiotics after 72 hours despite remaining unwell.10
Although most children are likely to obtain marginal benefit from immediate antibiotics in the short term, there are several unanswered issues: is there likely to be benefit in the longer term — particularly for outcomes important to parents — what factors predict adverse outcome, and whether ‘at risk’ subgroups are likely to benefit from treatment. We have reported the short-term results of a trial which compared the ‘wait and see approach’ with immediate antibiotics,11 and demonstrated that for most children there was marginal short-term benefit of immediate antibiotics with no significant difference in pain or distress scores — since what benefit there was from antibiotics mainly occurred after the first 24–48 hours when symptoms are milder.12 We now report the predictors of poor medium- and longer-term outcome from this trial cohort.
METHOD
This study was approved by several local research ethics committees. The methods12 and referencing of the discussion about the pragmatic diagnostic criteria we used have previously been reported in full elsewhere.1,12–17 Children were eligible if they were aged 6 months to 10 years, attending their doctor with acute otalgia and otoscopic evidence of acute inflammation, and using the same photographic examples of each physical sign to guide physicians as in a previous trial.13,17 Where children were too young to specifically document otalgia from the history (under 3 years old), then otoscopic evidence only was a sufficient entry criteria. The study design and trial flow are shown in Figure 1.
Figure 1.
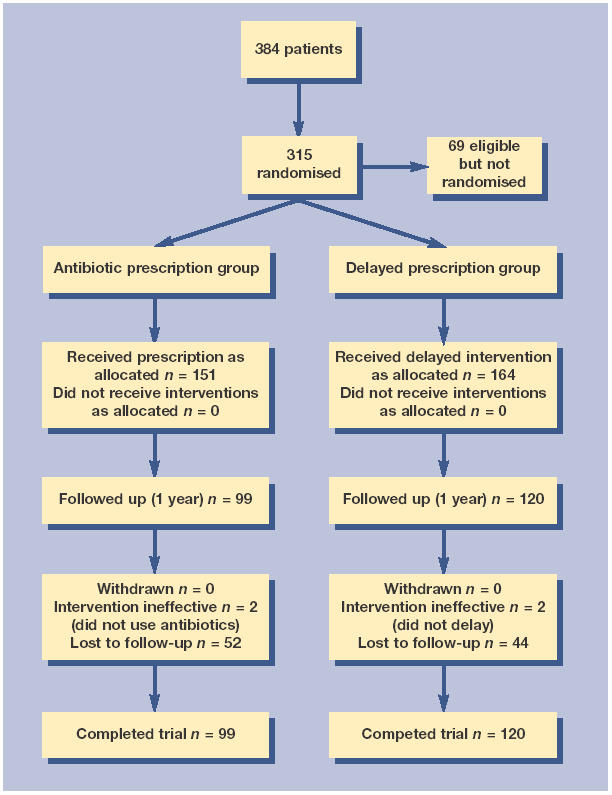
Study design and trial flow diagram.
Intervention
After written consent, patients were randomised when the doctor opened a sealed numbered opaque envelope containing a standardised advice sheet5,6,12 for one of two groups:
Immediate antibiotics: amoxicillin according to British National Formulary guidelines, and the same dosaging regime as the previous largest blinded trial from primary care13 (or erythromycin if allergic to penicillin);
Delayed antibiotics: as in (1), but patients were asked to wait for 72 hours after seeing the doctor before considering using the prescription. Parents were advised to use antibiotics if their child had significant otalgia or fever after 72 hours, or if discharge lasted for 10 days or more.
How this fits in
There is very limited data from previous randomised trials about longer-term outcomes in acute otitis media from either prescribing immediately or delayed prescribing. The delayed prescribing strategy did not significantly increase the risk of earache or poor function at 3 months or 1 year. Secondary analysis provides some weaker evidence that children with previous recurrent otitis media were at risk of poorer outcomes in the longer term. However the data from this study suggest that prescribing antibiotics is not likely to modify this.
Outcome measurement and other documentation
Prior episodes
Blind to randomisation group, a researcher extracted information about attendance with prior episodes of otitis media from the medical notes.
Questionnaire
A questionnaire was sent at 3 months and then at 1 year after study entry to parents, with second and third mailing to non-responders asking about earache and functioning. The emphasis was to measure outcomes likely to be important to parents:
Earache: the questionnaire asked parents whether there had been any further episodes of earache since study entry. We have previously shown evidence of the validity of parental rating of pain/earache.12 We report earache since study entry at both 3 months (that is, from randomisation to 3 months), and also 1 year (that is, from randomisation to 1 year);
Child functioning: the questionnaire also contained seven questions that parents scored about their child's language and social functioning: mishearing what is said; speech difficult to understand; shouts; has poor pronunciation; difficulty learning to read; poor concentration; and, is a daydreamer. These questions were a subset of questions from a previous study which provided 14 descriptions of how hearing impairment with chronic secretory otitis media presents:18 the seven questions used were shown in a sample of 140 school children to have good inter-rater reliability between parents and teachers — and were thus more likely to be clinically useful. Each question was scored on a 3-point Likert scale (0 = does not apply, 1 = sometimes applies, and 2 = definitely applies). Factor analysis of the seven questions in this cohort showed a one-factor solution explaining 90% of the variance with all items loading strongly onto the factor (loading 0.50 or more) except difficulty reading. Thus, in our analysis we used the six-item function scale based on a simple sum of the six questions (that is, all questions except difficulty reading): this scale has a Cronbachs's α value of 0.75 — in the optimal range,19 suggesting that it is reliable. Clearly the rating scale is not appropriate for children with little or no language (such as those under 2 years of age), but this trial cohort had very few very young children: by the 1-year follow-up only 3.5% were aged under 2 years, 10.5% under 3 years, and 24% under 4 years. A cut-off of 9 on the score represents a group of children performing poorly (the worst 20%), where their parents felt, on average, all items sometimes applied and at least three out of six items definitely applied.
Sample size (for 80% power and 95% confidence using the Epi Info sample size program)
The original sample size calculation was based on short-term outcomes:12 to detect a 15% difference in the number of children better by 72 hours after seeing their doctor required 233 children, or 291 children in total, allowing for up to 20% loss to follow-up. We recruited 315 children: we estimated that this sample size allowing for up to 30% non-response to follow-up questionnaires, would allow detection of risk factors for adverse outcomes with an odds ratio (OR) of 2.5 (that is, a significant risk factor) — assuming adverse outcomes occur in 50% of children (earache reported at 3 months or 1 year), and assuming the prevalence of risk factors varies from 20–65%.
Analysis
We assessed the effect of intervention and predictors of poor outcome using logistic regression for dichotomous outcomes. Variables significant in univariate analysis at the 5% level were entered by forward selection, starting with the most significant first, and retained if they remained significant at the 5% level. Variables that predicted poor outcome were then used to identify clinical subgroups, and the estimated effect of antibiotic in those subgroups was assessed by the χ2 test for dichotomous outcomes. To assess the effect of intervention for continuous outcomes we used the t test. Our primary analyses were the randomised comparisons, and the assessment of the role of prior episodes of otitis media in predicting subsequent episodes of otalgia. Our secondary analyses were the assessment of risk factors for poor function, and the assessment of benefit of antibiotics in the higher-risk subgroups. Secondary analyses should be treated with caution in view of the danger of type I error.
RESULTS
At 3 months and 1 year, responses were received from 223 (71%) and 219 (70%) participants, respectively. There was no difference in the characteristics of responders compared with non-responders in those receiving immediate antibiotics (47% and 51%, respectively; P = 0.55), vomiting (18% and 22% P = 0.54) prior episodes of otitis media (46% and 48% P = 0.94), discharge (26% and 26% P = 0.91), aged 3 years and under (42% and 36% P = 0.34) and with a bulging drum (46% and 49% P = 0.58). There was good agreement between reported collection of antibiotic prescriptions and actual collection, and good differentiation between antibiotic and delayed groups in the number who took an antibiotic prescription.12
The effect of prescribing strategies
The delayed prescribing strategy did not significantly increase risk of earache at 3 months (OR = 0.89; 95% confidence interval [CI] = 0.48 to 1.65) or 1 year (OR = 1.03; 95% CI = 0.60 to 1.78), or poor scores on the function scale at 3 months (OR = 1.37; 95% CI = 0.72 to 2.60) or 1 year (OR = 1.16; 95% CI = 0.61 to 2.23). Controlling for subsequent antibiotic use (that is, after the randomised episode) did not significantly alter the estimates for any of the above outcomes (OR = 0.85, 0.80, 1.43, 1.16, respectively). To check that the negative result for the function score could not be explained by the particular cut-off, we also assessed the function score as a continuous variable: the delayed prescribing strategy made very little difference at 3 months (β = 0.14; 95% CI = −0.68 to 0.39; P = 0.60) or 1 year (β = −0.05; 95% CI = −0.49 to 0.58; P = 0.87).
Predictors of poor outcome
Predictors of poor outcome are shown in Tables 1–4. Prior episodes of otitis media documented in the doctor's notes predicted episodes of earache reported since study entry after 1 year, and the secondary outcomes (weaker evidence) of parental rating of function at both 3 months and 1 year. Other factors that possibly predict poor outcome were bulging drum and ear discharge in the index episode, which predicted episodes of earache in the 3 months since study entry.
Table 1.
Predictors of episodes of earache reported after 3 months.
| Had earache n (%) | Did not have earache n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)a | LR χ2 (P-value) | |
|---|---|---|---|---|---|
| Symptoms and signs: | |||||
| High temperature | 11 (21) | 23 (14) | 1.66 (0.75 to 3.68) | 1.62 (0.71 to 3.71) | 1.25 (0.26) |
| Vomiting | 8 (16) | 32 (19) | 0.78 (0.34 to 1.83) | 0.63 (0.26 to 1.52) | 1.13 (0.29) |
| Ear discharge | 20 (39) | 35 (21) | 2.43 (1.24 to 4.78) | 2.57 (1.29 to 5.13) | 7.04 (0.004) |
| Bulging drum | 30 (58) | 71 (42) | 1.86 (0.99 to 3.50) | 2.18 (1.13 to 4.20) | 5.50 (0.019) |
| Past history — prior episodes of otitis media: | |||||
| 0 | 78 (53) | 26 (54) | 1 | 1 | 0.52 (0.77) |
| 1 | 37 (25) | 11 (23) | 0.89 (0.40 to 2.00) | 0.80 (0.34 to 1.84) | |
| 2 | 32 (22) | 11 (23) | 1.03 (0.46 to 2.33) | 0.75 (0.30 to 1.85) | |
| Family/social factors — child care: | |||||
| Playgroup | 13 (29) | 29 (22) | 1 | 1 | 0.43 (0.81) |
| Nursery | 4 (9) | 19 (14) | 0.47 (0.13 to 1.66) | 0.65 (0.17 to 2.45) | |
| School | 28 (62) | 86 (64) | 0.73 (0.33 to 1.59) | 0.83 (0.36 to 1.92) | |
OR = odds ratio. LR = likelihood ratio.
Adjusted for significant predictors of outcome. Other variable assessed in models and shown not to predict outcome: asthma, eczema, exposure to smoke in the home, educational status of parents, history of breast feeding, past and current use of dummy, sibling having had otitis media.
Table 4.
Predictors of poor score (9 or more) on function scale after 1 year.
| Had poor function n (%) | Did not have poor function n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)a | LR χ2 (P-value) | |
|---|---|---|---|---|---|
| Symptoms and signs: | |||||
| High temperature | 9 (19) | 28 (16) | 1.24 (0.54–2.86) | 1.53 (0.64–3.66) | 0.89 (0.35) |
| Vomiting | 12 (26) | 35 (21) | 1.35 (0.63–2.88) | 1.28 (0.59–2.78) | 0.38 (0.54) |
| Ear discharge | 18 (39) | 42 (25) | 1.94 (0.98–3.86) | 1.62 (0.79–3.32) | 1.67 (0.20) |
| Bulging drum | 23 (50) | 83 (47) | 1.11 (0.58–2.12) | 1.03 (0.52–2.02) | 0.01 (0.94) |
| Past history — prior episodes of otitis media: | |||||
| 0 | 17 (37) | 87 (57) | 1 | 1 | 4.56 (0.033) (test for trend) |
| 1 | 16 (35) | 38 (25) | 2.15 (0.99–4.71) | 2.15 (0.99–4.71) | |
| 2 | 13 (28) | 29 (19) | 2.29 (0.99–5.29) | 2.29 (0.99–5.29) | |
| Family/social factors — child care: | |||||
| Playgroup | 6 (20) | 27 (23) | 1 | 1 | 1.24 (0.54) |
| Nursery | 3 (10) | 18 (15) | 0.75 (0.17–3.39) | 0.61 (0.13–2.89) | |
| School | 21 (70) | 73 (62) | 1.29 (0.47–3.55) | 1.25 (0.44–3.58) | |
OR = odds ratio. LR = likelihood ratio.
Adjusted for significant predictors of outcome. Other variable assessed in models and shown not to predict outcome: asthma, eczema, exposure to smoke in the home, educational status of parents, history of breast feeding, past and current use of dummy, sibling having had otitis media.
Does treatment with antibiotics in subgroups with poor outcome predict response?
This was a secondary subgroup analysis and as such should be treated with caution. In children with recurrent otitis media (≥2 previous episodes) (n = 43), there was weaker evidence and fewer episodes of earache were reported in the 3 months since study entry (immediate antibiotics 10% delayed 39% χ2 4.8; P = 0.029). There was a similar effect for poor functioning at 3 months (20% compared to 44% χ2 2.69; P = 0.10). However, in the same group of children, immediate treatment with antibiotics did not predict outcome for parental rating of poor functioning at 1 year (30% and 32%) or episodes of earache reported since study entry at 1 year (47% and 52%).
DISCUSSION
Summary of main findings
This randomised controlled trial of antibiotic prescribing strategies for acute otitis media in typical primary care settings suggests that delayed prescribing does not result in adverse medium- to longer-term outcomes. Children with recurrent episodes are likely to have poorer outcome, and secondary analysis of this subgroup — which should be treated with caution (due to weaker evidence) — suggests that they may possibly benefit from immediate antibiotics in the medium term, but not in the longer term.
Strengths and limitations of this study
The main strengths of this study are the adequate long-term follow-up, the size of the study, the generalisable sample, and the pragmatic interventions and outcomes. Before discussing the detailed results, the potential limitations of this study will be outlined.
Selection and non-response bias
We have shown that low-recruiting doctors were apparently a little more unsure about recruiting younger children,12 and the cohort contained few very young children. Thus, although there was no evidence that age predicted outcome in this cohort, it is more difficult to extrapolate the results to very young children. There was no evidence of significant differences in the characteristics of those who responded to follow-up questionnaires and those who did not.
Placebo effect
An open trial design and minimally intrusive outcomes (for example, no intrusive measures of compliance, or investigation) was chosen to assess realistic outcomes in everyday practice, but has the important disadvantage of a potential placebo effect. Although we minimised this using a structured advice sheet approach — which has been shown to abolish the antibiotic placebo effect in a previous trial5 — a component of the placebo effect is possible. However, the effect if any is probably small: previous estimates from this study (for example, night disturbance and paracetamol consumption)12 were very similar to the largest blinded trial performed in a similar study population,13 and the estimates in this study demonstrate little or no placebo effect.
Outcomes
We wanted to measure outcomes reflecting parents' concerns — both of discomfort for their child and their child's functioning. Thus the study can be criticised for potential recall bias of episodes of earache, and by not including ‘hard’ outcomes such as tympanometry or audiometry — but the relationship of such outcomes to child discomfort, functioning and development is not clear-cut.20 We have shown that the outcomes chosen are likely to be reliable, they are important to parents, and they reflect likely impact on health service utilisation by parents.18
Subsequent antibiotic use
Subsequent antibiotic use after the randomised episode could potentially have altered the longer-term outcomes of the two prescribing strategies. In fact, controlling for subsequent antibiotic use did not alter the estimates.
Subgroup analysis
This study reports estimates of both the predictors of poor outcome, and also the effect of antibiotics in a clinical subgroup of a randomised trial (that is, a danger of type I error). These findings therefore require confirmation in further prospective studies.
Comparison with existing literature
Who is at risk of poor outcome?
The most consistent predictor of poor outcome was the number of prior episodes of otitis media documented in the doctor's notes, which predicted episodes of earache reported since study entry after 1 year, and parental rating of function at both 3 months and 1 year. This is consistent with previous evidence,21 which suggests that those with post-otitis media or secretory otitis media, past acute otitis media (AOM), and who were boys were less likely to have good outcome at 2 months.21 We were unable to confirm other risk factors previously identified for adverse outcome such as sibling ear infection, use of a dummy, and age.3
Can poorer medium and long-term outcomes be modified?
For most children using a delayed prescribing strategy is not likely to have adverse longer-term consequences. The current study suggests that medium-term outcome may possibly be modified in children with recurrent ear infections — but with the important caveat about type I error above for secondary subgroup analysis. Furthermore, when longer-term outcomes are included (that is, from randomisation to 1 year), there is no longer an effect. A systematic review suggests prophylaxis has minimal effects,22,23 but there is mixed evidence as to whether treating upper respiratory tract infections in otitis-prone children reduces subsequent otitis media in children with recurrent otitis media.24,25 The difficulty of modifying longer-term outcomes is supported by previous research: when compared to children with no AOM, children with recurrent AOM had worse thresholds only at high frequencies when followed up to age 7 years.26 The long-term outlook for most children with ear infections is good: adults who remember ear infections in childhood, are no more likely to suffer hearing impairment when compared to who do not remember ear infections.27
Implications for future research
Secondary analysis from this trial cohort highlights the importance of performing a larger randomised controlled trial to assess the benefit of antibiotics and alternatives to antibiotics in the medium to longer term for children with recurrent otitis media.
Table 2.
Predictors of episodes of earache reported after 1 year.
| Had earache n (%) | Did not have earache n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)a | LR χ2 (P-value) | |
|---|---|---|---|---|---|
| Symptoms and signs: | |||||
| High temperature | 18 (21) | 18 (13) | 1.73 (0.84 to 3.55) | 1.88 (0.85 to 4.17) | 2.40 (0.12) |
| Vomiting | 19 (24) | 26 (20) | 1.28 (0.66 to 2.50) | 1.45 (0.72 to 2.92) | 1.09 (0.30) |
| Ear discharge | 27 (34) | 32 (24) | 1.61 (0.87 to 2.96) | 1.40 (0.72 to 2.69) | 0.98 (0.32) |
| Bulging drum | 42 (50) | 63 (47) | 1.13 (0.65 to 1.95) | 1.01 (0.56 to 1.83) | 0.00 (0.98) |
| Past history — prior episodes of otitis media: | |||||
| 0 | 28 (37) | 73 (60) | 1 | 1 | 8.04 (0.005) (test for trend) |
| 1 | 26 (35) | 28 (23) | 2.42 (1.22 to 4.82) | 2.42 (1.22 to 4.82) | |
| 2 | 21 (28) | 21 (17) | 2.61 (1.24 to 5.49) | 2.61 (1.24 to 5.49) | |
| Family/social factors — child care: | |||||
| Playgroup | 14 (25) | 19 (21) | 1 | 1 | 1.52 (0.47) |
| Nursery | 7 (12) | 15 (15) | 0.68 (0.22 to 2.12) | 0.46 (0.13 to 1.62) | |
| School | 36 (63) | 58 (64) | 0.84 (0.38 to 1.89) | 0.79 (0.33 to 1.90) | |
OR = odds ratio. LR = likelihood ratio.
Adjusted for significant predictors of outcome. Other variable assessed in models and shown not to predict outcome: asthma, eczema, exposure to smoke in the home, educational status of parents, history of breast feeding, past and current use of dummy, sibling having had otitis media.
Table 3.
Predictors of poor score (9 or more) on function scale after 3 months.
| Had poor function n (%) | Did not have poor function n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)a | LR χ2 (P-value) | |
|---|---|---|---|---|---|
| Symptoms and signs: | |||||
| High temperature | 8 (16) | 26 (15) | 1.12 (0.47 to 2.65) | 0.96 (0.36 to 2.59) | 0.01 (0.94) |
| Vomiting | 13 (27) | 27 (16) | 1.93 (0.90 to 4.10) | 1.96 (0.88 to 4.40) | 2.58 (0.11) |
| Ear discharge | 18 (37) | 38 (22) | 2.03 (1.03 to 4.03) | 1.96 (0.94 to 4.11) | 3.12 (0.077) |
| Bulging drum | 22 (46) | 80 (46) | 0.99 (0.52 to 1.89) | 0.99 (0.49 to 1.97) | 10.0 (0.97) |
| Past history – prior episodes of otitis media: | |||||
| 0 | 17 (40) | 88 (58) | 1 | 1 | 4.95 (0.026) (test for trend) |
| 1 | 12 (28) | 36 (24) | 1.73 (0.75 to 3.98) | 1.73 (0.75 to 3.98) | |
| 2 | 14 (33) | 29 (19) | 2.50 (1.10 to 5.69) | 2.50 (1.10 to 5.69) | |
| Family/social factors — child care: | |||||
| Playgroup | 9 (21) | 34 (25) | 1 | 1 | 4.79 (0.091) |
| Nursery | 2 (5) | 21 (15) | 0.36 (0.07 to 1.83) | 0.31 (0.06 to 1.65) | |
| School | 31 (74) | 84 (60) | 1.39 (0.60 to 3.24) | 1.36 (0.54 to 3.38) | |
OR = odds ratio. LR = likelihood ratio.
Adjusted for significant predictors of outcome. Other variable assessed in models and shown not to predict outcome: asthma, eczema, exposure to smoke in the home, educational status of parents, history of breast feeding, past and current use of dummy, sibling having had otitis media.
Acknowledgments
We are grateful to the following doctors for their enthusiasm and help in recruitment: Doctors Newman, Taylor, Traynor, Tippett, Warner, Peace, Stephens, Glasspool, Stone, Webb, Snell, Devereux, Hoghton, Terry, Dickson, Nightingale, Richenbach, Bacon, Lupton, Padday, Cookson, Stanger, Glaysher, Bond, Baker, Barnsley, Jeffries, Willard, Carlisle, Hill, Collier, Cubitt, De Quincey, Over, White, Billington, Percival, Hollands, Glaysher, Stranger.
Funding body
Paul Little is supported by the Medical Research Council (G108/322). The current study was supported by a grant from regional NHS responsive mode funding in the South West region
Ethics committee
Isle of White and Portsmouth (LReC number 12/96/484) also North and Mid Hampshire, Salisbury, Southampton and South West Hampshire
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Bain J. Controversies in therapeutics. Childhood otalgia. Justification for antibiotic use in general practice. BMJ. 1990;300:1006–1007. doi: 10.1136/bmj.300.6730.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browning G. Controversies in therapeutics. Childhood otalgia: acute otitis media. Antibiotics not necessary in most cases. BMJ. 1990;300:1005–1006. doi: 10.1136/bmj.300.6730.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Froom J, Culpepper L, Jacobs M, et al. Antimicrobials for acute otitis media? A review from the international primary care network. BMJ. 1997;315:98–102. doi: 10.1136/bmj.315.7100.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Mar C, Glasziou P, Hayem M. Are antibiotics indicated as initial treatment for children with acute otitis media? A meta-analysis. BMJ. 1997;314:1526–1529. doi: 10.1136/bmj.314.7093.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little PS, Williamson I, Warner G, et al. An open randomised trial of prescribing strategies for sore throat. BMJ. 1997;314:722–727. doi: 10.1136/bmj.314.7082.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little PS, Gould C, Williamson I, et al. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350–352. doi: 10.1136/bmj.315.7104.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britten N, Ukoumunne O. The influence of patients' hopes of receiving a prescription on doctors' perceptions and the decision to prescribe: a questionnaire survey. BMJ. 1997;315:1506–1510. doi: 10.1136/bmj.315.7121.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacFarlane J, Holmes W, MacFarlane R, Britten N. Influence of patients' expectations on antibiotics management of acute lower respiratory illness in general practice: questionnaire study. BMJ. 1997;315:1211–1214. doi: 10.1136/bmj.315.7117.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arason V, Kristinsson K, Sigurdsson J, et al. Do antimicrobials increase the rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ. 1996;313:387–391. doi: 10.1136/bmj.313.7054.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Buchem FL, Peeters MF, van't Hof MA. Acute otitis media a new treatment strategy. BMJ. 1985;290:1033–1037. doi: 10.1136/bmj.290.6474.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitts J. Shared decision making in the informed treatment of acute otitis media. Practitioner. 1987;231:1232–1233. [PubMed] [Google Scholar]
- 12.Little P, Gould C, Williamson I, et al. A pragmatic randomised controlled trial of two prescribing strategies for acute otitis media. BMJ. 2001;322:336–342. doi: 10.1136/bmj.322.7282.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke P, Bain J, Robinson D, Dunleavey J. Acute red ear in children: controlled trial of non-antibiotic treatment in general practice. BMJ. 1991;303:558–562. doi: 10.1136/bmj.303.6802.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gates G, Northern J, Ferrer P, et al. Diagnosis and screening. Ann Otol Rhinol Laryngol. 1989;98(suppl 139):39–41. [PubMed] [Google Scholar]
- 15.Schwartz R, Rodriguez W, Brook I, Grundfast K. The febrile response in otitis media. JAMA. 1981;245:2057–2058. [PubMed] [Google Scholar]
- 16.Preston K. Pneumatic otoscopy: a review of the literature. Issues In Compr Pediatr Nurs. 1998;21(2):117–128. doi: 10.1080/014608698265537. [DOI] [PubMed] [Google Scholar]
- 17.Ruuskanen O, Heikkinen T. Otitis media: etiology and diagnosis. Ped Inf Dis J. 1994;13:s23–s26. [PubMed] [Google Scholar]
- 18.Maw A, Tiwari R. Children with glue ear: how do they present? Clin Otolaryngol Rhinol. 1988;13:171–177. doi: 10.1111/j.1365-2273.1988.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 19.Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. Oxford: Oxford Medical Publications; 1995. [Google Scholar]
- 20.Williamson I, Sheridan C, Galker E, Lous J. A video-based performance in noise test for measuring audio-visual disability in young school children: test development, with validation by trained teachers, parents and audiometry as relative standards for disability. Int J Pediatr Otorhinolaryngol. 1999;49:127–133. doi: 10.1016/s0165-5876(99)00110-x. [DOI] [PubMed] [Google Scholar]
- 21.Froom J, Culpepper L, Bridges-Webb C, et al. Effect of patient characteristics and disease manifestations on the outcome of acute otitis media at 2 months. Arch Fam Med. 1993;2:841–846. doi: 10.1001/archfami.2.8.841. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill P. Otitis media: a clinical review. BMJ. 1999;319:833–835. doi: 10.1136/bmj.319.7213.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantekin E. Where is the evidence? [rapid response] BMJ. 1999;319:833–835. [Google Scholar]
- 24.Prellner K, Fogle-Hansson M, Jorgensen F, et al. Prevention of recurrent acute otitis media in otitis-prone children by intermittent prophylaxis with penicillin. Acta Otorhinolaryngol. 1994;114:182–187. doi: 10.3109/00016489409126039. [DOI] [PubMed] [Google Scholar]
- 25.Autret-Leca E, Giraudeau B, Ployet M, Jonville-Bras A. Amoxicillin/clavulanic acid is ineffective at preventing otitis media in children with presumed viral upper respiratory infection: A randomised, double-blind equivalence, placebo-controlled trial. Br J Clin Pharmacol. 2002;54:652–656. doi: 10.1046/j.1365-2125.2002.t01-6-01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorri M, Maki-Torkko E, Alho O. Otitis media and long term follow-up of hearing. Acta Otorhinolaryngol. 1995;115:193–195. doi: 10.3109/00016489509139290. [DOI] [PubMed] [Google Scholar]
- 27.Stephenson H, Higson J, Haggard M. Binaural hearing in adults with histories of otitis media in childhood. Audiology. 1995;34:113–123. doi: 10.3109/00206099509071905. [DOI] [PubMed] [Google Scholar]


