Abstract
The ventromedial hypothalamus (VMH) is one of several sexually dimorphic nuclei that regulate mating behavior, and is rich in steroid hormone receptors and aromatase activity. We looked at the contribution of the androgen receptor (AR) to the volume of the VMH in rats by measuring each of the four subdivisions of the VMH in 90 day old male, female, and XY male rats carrying a mutant AR allele (tfm), which renders animals largely unresponsive to androgens. Confirming published reports, total VMH volume was greater in wild-type males than in females (p < 0.01). The mean total volume of the VMH in TFM males was intermediate, but not significantly different from either females or males (Ps > 0.10). The sex difference in VMH volume was primarily accounted for by the ventrolateral subdivision (VMHvl), which in both females and TFM males was significantly smaller than in wild-type males (Ps < 0.005). There was no significant sex difference in the volume of the other three subdivisions of the VMH. Neuronal somata were larger in males than females in VMHvl, central VMH (VMHc) and the dorsomedial VMH (VMHdm), with TFM males having feminine neuronal somata in the VMHdm and VMHc. These data suggest that AR plays a role during sexual differentiation of the VMH, imparting its greatest effect in the VMHvl. ARs may regulate aromatase expression or activity to affect estrogen receptor activation, or may act independently of estrogen receptors to influence VMH morphology.
Keywords: Tfm, Sex differences, Sexual dimorphism, Androgen sensitivity, Laterality, Brain
Introduction
The ventromedial hypothalamus (VMH) is one of several sexually differentiated nuclei in the tuberal area of the hypothalamus (Simerly, 1995) and presents an oval profile in coronal sections in rodents. The VMH has been implicated in several sexually dimorphic behaviors, including parental and sexual behaviors (Etgen and Morales, 2002; Yahr and Greene, 1992; Flanagan-Cato, et al., 2001; Flanagan-Cato et al., 2006; Harding and McGinnis, 2005; Kow and Pfaff, 1998; Pfaff and Sakuma, 1979; Matsumoto and Yamanouchi, 2000).
The VMH is comprised of four distinct cell condensations: the anterior (VMHa), dorsomedial (VMHdm), ventrolateral (VMHvl) and central (VMHc), which not only differ anatomically and neurochemically, but also behaviorally. The VMHvl and VMHdm contain high concentrations of steroid receptor mRNAs, and are the most studied of the four subdivisions (Simerly, et al., 1990; Wagner and Morrell, 1996). For example, the VMHdm appears to be critical for the expression of ultrasonic vocalization and scent marking behaviors in males (Yahr and Greene, 1992; Flanagan-Cato et al., 2001; Harding and McGinnis, 2005). In females, lesioning of the VMHdm stimulates fast-latency maternal behaviors (Mann and Babb, 2004). On the other hand, the VMHvl appears to play a role in the expression of sex behaviors in both sexes--lordosis behavior in females (Kow and Pfaff, 1998), and mounting behavior in males (Christensen, et al., 1977; Pfaff and Sakuma, 1979; Matsumoto and Yamanouchi, 2000).
These observations raise the question of how the same nucleus in males and females can be responsible for such gender-specific behaviors and indeed, whether the various subnuclei within the VMH vary in their organization or degree of sexual dimorphism. Although volume of the VMH is reportedly sexually dimorphic, with males having significantly larger overall volume than females (Matsumoto and Arai, 1983), it is not known whether this sex difference is present in all four nuclei of the VMH. Moreover, while the structural components potentially contributing to overall differences in volume have begun to be elucidated—males have both larger neuronal somata and more neuropil than females (Madeira, et al., 2001)—again, such sex differences have not been characterized within each subnucleus of the VMH.
Another question of particular interest is whether the androgen receptor (AR) contributes to sex differences in volume and soma size in the VMH. The VMH contains a high concentration of ARs (McGinnis, et al., 1983; Simerly et al., 1990) with significantly higher expression in males than in females (Roselli, 1991) paralleling similar sex differences in androgen binding (McGinnis and Katz, 1996). Such sex differences in AR complement raises the possibility that ARs may be critically involved in determining sex differences in VMH morphology and function. Some data support this view. For example, pharmacologically blocking ARs in the VMH of adult male rats inhibits copulation (Harding and McGinnis, 2004). However, there is no published data to date on whether ARs, either in the VMH or elsewhere, have a role in its sexual differentiation. The present study addresses this question.
We studied male XY rats that have the tfm (testicular feminization mutation) allele (TFM males), a mutant allele of the AR that renders males largely unresponsive to androgens (Yarbough, et al., 1990). Since the AR gene is on the X chromosome, the tfm allele is passed from carrier females to XY offspring. In rats, testicular development and testosterone secretion appear to be normal in TFM males, but somatic tissues show little or no response to androgens. TFM males do not display lordosis despite their feminine appearance (Olsen, 1979), so they are defeminized, presumably by aromatized metabolites of testosterone acting on estrogen receptors. They also show infrequent and incomplete male copulatory responses to receptive females (Beach and Buehler, 1977), but that could be due to either incomplete masculinization of brain sites such as the VMH or to the absence of normal male genitalia.
In the present study, the role of ARs in sexual differentiation of the VMH was examined by comparing the volume and neuronal soma size in the four subnuclei of the VMH in adult TFM males to that of wild-type (WT) males and females. Results indicate that ARs normally play a role in masculinizing the VMH of rats, and most of the sexual dimorphism in VMH volume is accounted for by the VMHvl subregion.
Methods
Animals
Animals used in this study were a subset of the Long Evans rats used in a previous report (Morris, et al., 2005). Ninety day old wild type males (n=6), females (mixture of WT and tfm carriers n=10) and TFM males (n=8) were used, favoring littermates whenever possible. Animal care followed the standards of the National Institutes of Health, and all procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University. Animals were sacrificed with an overdose of sodium pentobarbital (120mg/kg, ip) and body weights and anogenital distance (AGD) were measured. Deep anesthesia was noted by lack of reflexes to tail and foot pinch as well as lack of a corneal reflex. Animals were then perfused transcardially with 0.9% saline, followed by a 10% neutral buffered formalin (approximately 300mL/animal). Phenotype was confirmed by examining body markers: only TFM males had nipples and short AGDs (reflecting low AR activation) in combination with testes (reflecting Y chromosome activity). Brains were removed and postfixed in buffered formalin for at least a month.
Histology
Brains were placed in a 20% 0.1M phosphate buffered sucrose solution (pH 7.4) at 4°C overnight prior to slicing. Brains were scored along the left cortex to mark laterality, blocked at the cerebellum and olfactory tubercle, and coronally sliced at a 40um setting on a freezing sliding microtome. Every third section was collected in a phosphate buffer (PGT - 0.1MPO4, 0.1% gelatin, 0.3% Triton; pH 7.4) and mounted onto gel-subbed glass slides. Mounted tissue was stained with thionin and coverslipped with Permount.
Microscopy
Volume
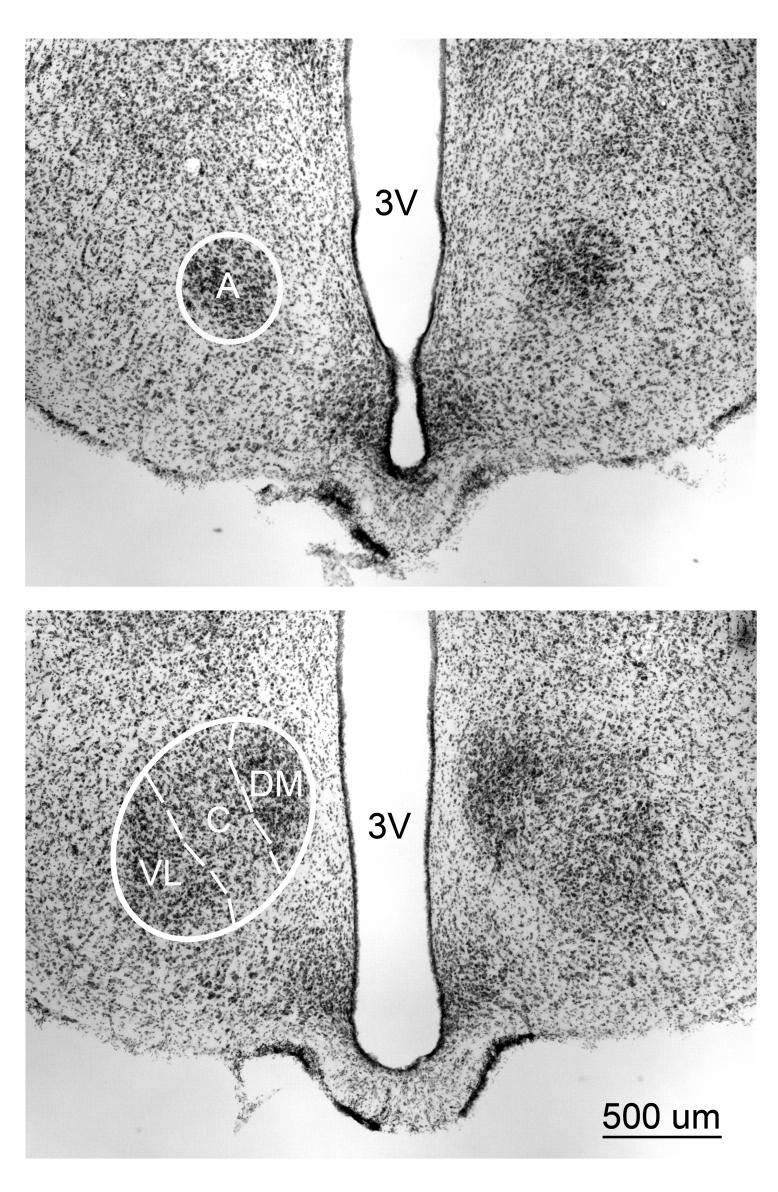
A Zeiss Axioplan2 Microscope along with Stereo Investigator Software (Microbrightfield, Colchester, VT) was used to analyze the tissue. An experimenter blind to each animal's treatment traced VMH cross-sectional area using a 5x objective and these traces were used to reconstruct volume using NeuroExplorer Software (Microbrightfield). Total cross sectional area was multiplied by the section thickness (40 um) and interval (3). Each of the four subdivisions of the VMH was traced rostral to caudal, beginning with the VMHa and ending with the VMHvl. The borders of each subdivision (Figure 1) were determined according to a standard rat atlas (Paxinos and Watson, 1998) and using published criteria from previous reports (Madeira et al., 2001), (Simerly, 1995). Total VMH volume for each animal was the summed volume of each of the four subnuclei.
Figure 1.
Thionin-stained 40µm coronal section taken at two different rostrocaudal levels of the VMH of an adult WT male. (Top) Rostral most appearance of the anterior portion (A) (Bottom) Further caudally in the same material showing the Dorsomedial, (DM) Central (C), and the Ventrolateral (VL) portions of the VMH appear. The dashed lines separate the divisions. 3V = Third ventricle.
Neuronal Soma Size
An experimenter blind to each animal's treatment measured neuronal soma size in the VMHa, VMHdm, VMHc and the VMHvl. Four or five neuronal somata were sampled on each side of each section per subdivision throughout the rostrocaudal extent of the nucleus using a 63X objective (range of 56-108 somata per animal per subdivision). The fractionator probe (Stereo Investigator) was used for systematic unbiased sampling of neurons. Neurons were distinguished by their large size, prominent cytoplasm and nucleolus. Neurons with the clearest borders closest to points randomly placed by the software were traced to provide a two-dimensional profile of soma size.
Statistics
Group differences in body weights and AGD were assessed using one-way analyses of variance (ANOVA). Genotype and laterality effects on volume and somal area measures were assessed using general linear model analysis of variance (SPSS version 12.0), with “Laterality” as a within-subjects factor (2 levels: left and right side) and “Genotype” (3 levels: control male, control female, and TFM male) as a between-subjects factor. Tukey post hoc tests were used to identify sources of variance. For volume, the VMH as a whole was analyzed, and then each subdivision was analyzed independently. For soma size, group comparisons were made only within each VMH subnucleus. All tests were two-tailed and considered statistically significant if p < 0.05.
Results
Body weight and Anogenital Distance
As previously reported with a different cohort of these animals (Morris et al. 2005), body weights of TFM males were significantly lighter than those of WT males and significantly heavier than WT females (Ps < 0.001 for all, Table 1). Similarly, AGD in TFM males was significantly different from both males and females (Ps < 0.001), but were closer to those of females than of WT males.
Table 1.
Body weight and anogenital distance of subjects. Average body weights and anogenital distance (AGD) for each group, with standard errors of the means in parentheses. All groups are significantly different from each other (1-way ANOVA for each measure, post-hoc tests indicate all ps<0.001).
| Group | Body Weight (g) | AGD (mm) |
|---|---|---|
| Males (n=6) |
515.8 (±22.6) | 44.1 (± 1.1) |
| TFMs (n=8) | 350.0 (± 10.3) | 18.3 (± 1.2) |
| Females (n=10) |
291.0 (± 10.7) | 13.0 (± 0.4) |
Volume
Effects of genotype: There was a main effect of genotype on VMH total volume (F=4.60 P < 0.022, Figure 2). Post hoc tests revealed that WT males had significantly greater VMH volumes than did females (P < 0.017; Figure 2), while VMH volume in TFM males was intermediate between WT males and females but not significantly different from either (Ps > 0.18).
Figure 2.
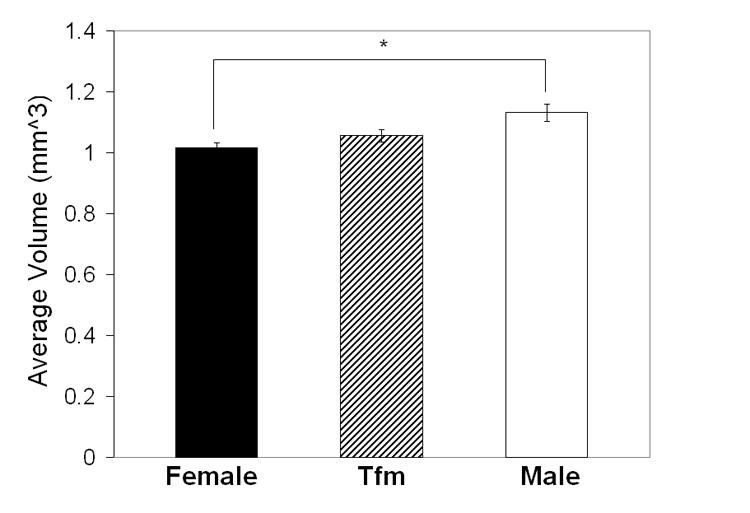
Mean (± SEM) total (bilateral) volume of the VMH in adult TFM male, WT male and female rats. VMH volume is significantly greater in WT males than in females. VMH volume in TFM males is intermediate and not significantly different from WT males or females. * P < 0.02.
This difference in total VMH volume across the groups was largely accounted for by differences in the VMHvl subdivision (F= 13.0 P < 0.0002, Figure 3). Volume of this subdivision was significantly larger in WT males than in females (P < 0.001) or TFM males (P < 0.004). VMHvl volume of TFM males was not different from that of females (P = 0.44). Although there was no main effect of genotype in VMHdm volume, examination of means led us to conduct t-tests, which suggested a marginal sex difference in the VMHdm (WT males > females; P = 0.07; Figure 3). VMHdm of TFM males were intermediate in volume between WT males and females without being significantly different from either. There were no group differences found in volume of VMHa or VMHc subdivisions. In sum, the sex difference in VMH volume is largely accounted for by the VMHvl subdivision with perhaps a contribution from the VMHdm. Laterality of volume: There was no significant main effect of laterality in total VMH volume or within individual subdivisions (Ps > 0.50). Nor was there a significant interaction between genotype and laterality. However, examination of means (Table 2) led us to conduct paired t-tests within each group for VMHdm and VMHvl. These tests indicated that right VMHdm volume is greater than the left in TFM males only (P < 0.02 Table 2). VMHvl volume was slightly greater on the left than the right in TFM males and WT males although this laterality was only marginally significant (P =0.053 and P = 0.06 respectively).
Figure 3.
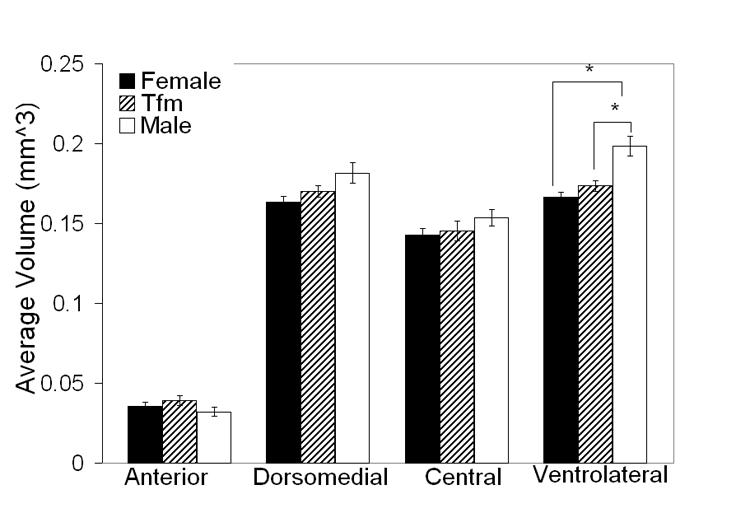
Mean (± SEM) bilateral volume of the four subdivisions of the VMH in adult TFM male rats compared with WT male and female littermates. Volume is significantly greater in WT males than in females only in the VMHvl. This subdivision is also larger in WT males than in TFM males. TFM males did not significantly differ from females in any of these measures. * P < 0.01.
Table 2.
Mean (±SEM) VMH regional volumes and soma areas of right and left hemispheres of adult TFM male rats and WT male and female littermate controls in each subdivision of the VMH. VMHdm right volume was significantly greater than VMHdm left volume only in TFM males. VMHvl left volume was marginally greater than VMHvl right volume in TFM males and WT males.
|
Females (n = 10) |
Tfms (n = 8) |
Males (n = 6) |
||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| VOLUME (mm^3 ×10^−3) | ||||||
| Anterior | 34.8+/− 3.5 | 36.1+/−3.7 | 35.9+/−3.7 | 42.1+/−4.8 | 31.6+/−5.0 | 32.8+/−3.9 |
| Ventrolateral | 166.6+/−4.8 | 165.9+/−4.6 | 180.8+/−3.6(†) | 166.4+/−4.3 | 206.7+/−9.0 (†) | 190.2+/−7.1 |
| Central | 142.6+/−6.0 | 142.9+/−5.3 | 146.7+/−10.0 | 144.0+/−7.8 | 153.1+/−4.8 | 153.9+/−9.5 |
| Dorsomedial | 162.1+/−5.2 | 164.3+/−5.4 | 165.6+/−5.6 (*) | 174.4+/−4.4 | 178.2+/−9.1 | 185.1+/−10.1 |
| SOMA (um^2) | ||||||
| Anterior | 172.7+/−4.1 | 180.6+/−2.8 | 179.1+/−2.3 | 172.8+/−3.2 | 184.6+/−3.9 | 185.2+/−3.7 |
| Ventrolateral | 190.9+/−2.1 | 192.0+/−2.3 | 195.3+/−4.18 | 192.9+/−5.2 | 207.3+/−4.2 | 203.6+/−3.4 |
| Central | 173.2+/−2.9 | 172.9+/−2.4 | 172.8+/−2.0 | 175.3+/−1.8 | 180.4+/−3.2 | 184.7+/−3.0 |
| Dorsomedial | 187.0+/−2.8 | 191.8+/−2.4 | 194.3+/−4.8(†) | 193.8+/−4.7 | 207.5+/−3.5 | 209.0+/−3.1 |
Marginally larger than right hemisphere (P=0.05 for Tfms and P=0.06 for Males)
Significantly smaller than right hemisphere (P < 0.02)
Neuronal Soma Size
There was a main effect of genotype on average soma cross sectional areas in the VMHvl (F=4.37 P < 0.03), VMHc (F= 4.05 P < 0.03) and VMHdm (F=7.55 P < 0.003) but not in the VMHa (F=2.59 P = 0.099). There was however a significant interaction of laterality and genotype for soma size in this subdivision (P<0.03). Paired t-tests to probe this interaction of laterality and genotype on soma size in the VMHa indicated that in fact there was no significant laterality of VMHa soma size in any of the three groups (all Ps > 0.1). There was neither a main effect of laterality nor an interaction of laterality and genotype for any other subdivision. Therefore we collapsed across sides to compare mean bilateral soma size across genotypes within each subnucleus. Soma cross-sectional areas were significantly smaller in females than in WT males in the VMHvl, VMHc and VMHdm (Ps < 0.02, 0.03 and 0.003 respectively; Figure 4). Neuronal somata in TFM males were also significantly smaller than in WT males in the VMHdm (P < 0.03), but were only marginally smaller in the VMHvl and VMHc (Ps = 0.08, two-tailed; Figure 4). Soma size in TFM males was not significantly different from females in any VMH subdivision (Ps > 0.50).
Figure 4.
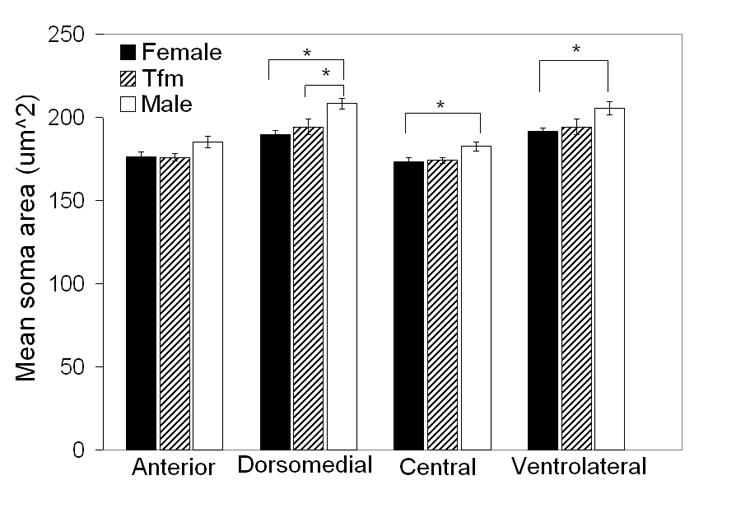
Mean (± SEM) cross-sectional area of neuronal somata in the VMHa, VMHvl, VMHc, and the VMHdm in adult TFM male rats and WT male and female littermates. Neuronal somata are larger in males than females in the VMHvl, VMHc and the VMHdm. TFM males have significantly smaller somata than their WT male littermates in the VMHdm, and marginally smaller somata in the VMHvl and VMHc. * P < 0.05.
Effect Sizes
The magnitude of sex differences in the VMH is not large in proportional terms: VMHvl volume is about 20% larger in males than in females; VMHvl somata are about 7% larger in males than in females. However, in terms of effect size, the sex differences appear robust, as d' (the difference in the two means divided by the average standard deviation from the two samples) is greater than 1.5 in both cases.
Discussion
Sex differences
We confirmed the previously reported sex difference in VMH volume in rats, finding that total VMH volume is greater in males than in females (Matsumoto and Arai, 1983; Madeira et al., 2001). We expanded our analysis to determine that the various subdivisions of the VMH differed in the extent of sexual dimorphism displayed, and that neuronal soma size was also dimorphic. The greatest volumetric sex difference is in the VMHvl, followed by a marginal sex difference in the VMHdm. In contrast, we found no evidence of a sexual dimorphism in volumes of the central or anterior subdivisions of the VMH (Figure 3).
As stated earlier, other research has suggested that both the VMHvl and VMHdm display neurochemical sex differences and have distinct behavioral roles. The VMHvl and VMHdm are both targets for gonadal steroids, but differ in the proportion of cells expressing ER and AR mRNA (Simerly et al., 1990; Devidze, et al., 2005). Data from the current study suggest that VMHvl and VMHdm volume may underlie some of these sex differences in function, since they are sexually dimorphic in this regard. Another cellular attribute that might also contribute to functional sex differences in the VMH is the size of neuronal somata. We report for the first time that neurons are larger in males than in females in three of the four subdivisions. This result agrees with a previous report that mean VMH soma size is larger in males than in females (Madeira et al., 2001).
In genetic male rats carrying the Tfm allele of the AR gene, both volume and neuronal soma size in were found to be less masculine than in WT males in at least one subdivision of the VMH. Total VMH volume in TFM males was intermediate between that of WT males and females but was not significantly different from either. However, examining individual subnuclei within the VMH indicated that volume of the VMHvl in TFM rats was significantly smaller than that of WT males but comparable in size to that of females. Thus, TFM males are incompletely masculinized in overall VMH volume and fully feminine in terms of VMHvl volume. Taken together, these data indicate that a functional AR is required for full masculinization of the VMH. This idea is reinforced by measurements of soma size in the VMHvl, VMHc and VMHdm in which TFM males do not differ from females but are significantly smaller than in WT males.
We found little evidence of lateral asymmetry in the VMH. The VMHvl in TFM and WT males had a slightly greater mean volume on the left than on the right, while the VMHdm was significantly larger on the right than the left in only TFM males. Females showed no laterality of VMH volume in any subdivision. These results indicate that volumetric laterality could be a masculine characteristic independent of AR in the VMHvl, since TFM males follow a masculine trend in the VMHvl. MacLusky and colleagues reported no significant right-left asymmetry in ER distribution between males and females in the VMH and several other regions, including the preoptic area and the bed nucleus of the stria terminalis (MacLusky, et al., 1997). There is a report of asymmetry of aromatase activity in the preoptic area of males (left > right) (von Ziegler and Lichtensteiger, 1992). For the VMHvl, this leaves the possibility that there could be asymmetries in aromatase activity that cause morphological differences in the two hemispheres. Since TFM males were the only group to display asymmetry in VMHdm, ARs in the VMHdm may promote lateral symmetry in volume of the VMHdm. Asymmetries in responsiveness to gonadal steroid, and in the expression of steroid receptors have been noted perinatally and in adulthood (Nordeen and Yahr, 1983; Xiao and Jordan, 2002; Cooke, et al., 2003).
Possible mechanism
There are several possible mechanisms by which ARs might promote masculinization of the VMH. Studies on another sexually differentiated region of the brain, the posterodorsal medial amygdala (MePD) in some ways parallel the present observations in the VMH. Like the VMH, both the volume of the MePD and the size of MePD neuronal somata are larger in males than in females, with TFM males having intermediate volumes and neuronal sizes (Morris et al., 2005). It does not seem likely that the partial masculinization of the MePD in TFM males reflects reduced activation of estrogen receptors (MacLusky et al., 1997), since neither testosterone levels nor aromatase activity in the medial amygdala of TFM males are reduced compared to WT males (Roselli, et al., 1987). However, in the VMH aromatase activity levels are lower in TFM males compared to WT males (Roselli, et al. 1987), so a reduction in aromatase activity may underlie demasculinization of the VMH in TFM males.
That ER mRNA is much higher in the VMHvl than the VMHdm (Simerly et al., 1990) implicates ER in masculinization of the most dimorphic subdivision of the VMH. The fact that testosterone treatment of adult male rats increases aromatase activity in several brain regions, including theVMH, compared to testosterone treated females (Roselli, 1991) also suggests that one role ARs might have in sexual differentiation of the VMH is to upregulate expression of aromatase, thus providing more aromatized testosterone to activate ER. It is also possible that activation of AR increases VMH volume and/or soma size via mechanisms entirely independent of aromatase and ER.
A related question is whether loss of AR function within the VMH or elsewhere is responsible for a less masculinized VMH in TFM animals. It is possible that the absence of functional AR in some other brain region led, via any of several possible pathways including changes in neuronal activity, neurotrophic factor release, etc., to the decrease in VMH volume and soma size. While local blockade of ARs within the VMH impairs male copulatory behavior (Harding and McGinnis, 2004), there is no data to date about whether such treatments also alter VMH volume and/ or neuronal somata size.
Provisos and cautions
Whenever a sexual dimorphism in the volume of a brain region is found, one question that normally arises is whether the dimorphism of the region simply reflects a sexual dimorphism of the entire brain. Indeed, we find that in general adult male rat brains weigh more than female brains. However, that cannot explain the pattern of dimorphisms we see in the VMH, because two of the four subdivisions (central and anterior) are sexually monomorphic in volume. So the dimorphism in the VMH is not general, but specific to two of the four subdivisions. Furthermore, while those subdivisions of the VMH in TFM males are generally feminine, unpublished observations from our lab have noted overall brain weight in TFM rats to be fully masculine, again indicating that VMH dimorphism reflects a specific rather than a general effect.
Another factor that was not controlled in our study is the female reproductive cycle. Physiological levels of gonadal steroids across the estrus cycle influence the morphology of VMHvl neurons (Sa and Madeira, 2005). Because we did not take into account the phase of estrus in females at the day of sacrifice, this may represent an additional source of variance for that group.
There is also the question of when during the life span AR activation regulates the masculinization of the VMH. The TFM allele produces a dysfunctional AR protein throughout the animals' lifetime, so we do not know whether functional AR is needed solely in the perinatal period, solely in the adult period, or in some combination of developmental periods, to fully masculinize the VMH. There is also the question of how a dysfunctional AR affects circulating levels of androgens such as testosterone at any point in the lifespan. A change in circulating testosterone might affect availability of aromatized metabolites to interact with ERs in the VMH. We know that circulating testosterone levels in adult TFM rats are, if anything, slightly higher than that of WT males (Roselli et al., 1987) (unpublished observations). So the demasculinization of the VMH cannot be due to lack of circulating testosterone, and therefore loss of substrate to be aromatized into estrogens to act upon ERs, in adulthood. We also know that the perinatal testes of TFM males secrete some hormone, because Olsen (Olsen, 1979) found that neonatal castration of TFM rats averted the defeminization of their brains as measured by the propensity to display lordosis behavior. But we do not know whether the perinatal testes of TFM males produce as much androgen as those of WT males. If they do not, then the dysfunctional Tfm allele of the AR gene may affect VMH morphology by affecting androgen secretion from the testes perinatally.
Further directions
It appears that androgens acting upon AR promote the masculinization of both VMH volume and neuronal soma size. Future studies may address when during development steroid hormones affect VMH morphology and whether such effects also involve aromatase. Although classical views of neural sexual dimorphism would suggest that perinatal steroid exposure “organizes” VMH structure in a permanent fashion, the finding that some brain regions, such as the MePD, can be sex-reversed by reversing the sex difference in circulating testosterone in adulthood, suggests that it is an open question of whether dimorphism in the VMH is due to organizational or “activational” influences of steroids. Another interesting experiment would be to examine the VMH of rats that are mosaic for AR, some cells expressing the WT allele while others express the Tfm allele, to ask whether only those VMH neurons directly producing functional AR are enlarged. If so, that would suggest that this is a cell-autonomous response of VMH neurons to androgen.
Acknowledgements
This work was supported by NIH grant MH58703.
Special thanks to Damian Zuloaga, David A. Puts, Erich N. Ottem and Cindy K. Knaff for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Current address: Department of Neuroscience, Michigan State University 108 Giltner Hall, East Lansing, MI 48824-1101, USA
References
- Beach FA, Buehler MG. Male rats with inherited insensitivity to androgen show reduced sexual behavior. Endocrinology. 1977;100(1):197–200. doi: 10.1210/endo-100-1-197. [DOI] [PubMed] [Google Scholar]
- Christensen LW, Nance DM, Gorski RA. Effects of hypothalamic and preoptic lesions on reproductive behavior in male rats. Brain Res Bull. 1977;2(2):137–41. doi: 10.1016/0361-9230(77)90010-7. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43(2):336–46. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Devidze N, Mong JA, Jasnow AM, Kow LM, Pfaff DW. Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proc Natl Acad Sci U S A. 2005;102(40):14446–51. doi: 10.1073/pnas.0507144102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Morales JC. Somatosensory stimuli evoke norepinephrine release in the anterior ventromedial hypothalamus of sexually receptive female rats. J Neuroendocrinol. 2002;14(3):213–8. doi: 10.1046/j.0007-1331.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Calizo LH, Daniels D. The synaptic organization of VMH neurons that mediate the effects of estrogen on sexual behavior. Horm Behav. 2001;40(2):178–82. doi: 10.1006/hbeh.2001.1679. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Lee BJ, Calizo LH. Co-localization of midbrain projections, progestin receptors, and mating-induced fos in the hypothalamic ventromedial nucleus of the female rat. Horm Behav. 2006;50(1):52–60. doi: 10.1016/j.yhbeh.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiol Behav. 2004;81(4):671–80. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Microlesions of the ventromedial nucleus of the hypothalamus: Effects on sociosexual behaviors in male rats. Behav Neurosci. 2005;119(5):1227–34. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res. 1998;92(2):169–80. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Bowlby DA, Brown TJ, Peterson RE, Hochberg RB. Sex and the developing brain: suppression of neuronal estrogen sensitivity by developmental androgen exposure. Neurochem Res. 1997;22(11):1395–414. doi: 10.1023/a:1022027408234. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432(3):329–45. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- Mann PE, Babb JA. Disinhibition of maternal behavior following neurotoxic lesions of the hypothalamus in primigravid rats. Brain Res. 2004;1025(12):51–8. doi: 10.1016/j.brainres.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 1983;30(3):277–80. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yamanouchi K. Acceleration of mounting behaviors in female rats by ibotenic acid lesions in the ventromedial hypothalamic nucleus. Neurosci Lett. 2000;291(3):143–6. doi: 10.1016/s0304-3940(00)01388-4. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Davis PG, Meaney MJ, Singer M, McEwen BS. In vitro measurement of cytosol and cell nuclear androgen receptors in male rat brain and pituitary. Brain Res. 1983;275(1):75–82. doi: 10.1016/0006-8993(83)90418-3. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Katz SE. Sex differences in cytosolic androgen receptors in gonadectomized male and female rats. J Neuroendocrinol. 1996;8(3):193–7. doi: 10.1046/j.1365-2826.1996.04494.x. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487(2):217–26. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Yahr P. A regional analysis of estrogen binding to hypothalamic cell nuclei in relation to masculinization and defeminization. J Neurosci. 1983;3(5):933–41. doi: 10.1523/JNEUROSCI.03-05-00933.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen KL. Androgen-insensitive rats are defeminized by their testes. Nature. 1979;279:238–239. doi: 10.1038/279238a0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–10. [PMC free article] [PubMed] [Google Scholar]
- Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128(3):1310–6. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121(6):2205–10. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- Sa SI, Madeira MD. Estrogen modulates the sexually dimorphic synaptic connectivity of the ventromedial nucleus. J Comp Neurol. 2005;484(1):68–79. doi: 10.1002/cne.20451. [DOI] [PubMed] [Google Scholar]
- Simerly RB. The Rat Nervous System second edition. Academic Press Inc; 1995. Anatomical Substrates of Hypothalamic Integration; pp. 353–376. [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- von Ziegler NI, Lichtensteiger W. Asymmetry of brain aromatase activity: region- and sex-specific developmental patterns. Neuroendocrinology. 1992;55(5):512–8. doi: 10.1159/000126165. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370(1):71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Horm Behav. 2002;42(3):327–36. doi: 10.1006/hbeh.2002.1822. [DOI] [PubMed] [Google Scholar]
- Yahr P, Greene SB. Effects of unilateral hypothalamic manipulations on the sexual behaviors of rats. Behav Neurosci. 1992;106(4):698–709. doi: 10.1037//0735-7044.106.4.698. [DOI] [PubMed] [Google Scholar]
- Yarbough WG, Quarmby VE, Simertal JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A Single Base Mutation in the Androgen Receptor Gene Causes Androgen Insensitivity in the Testicular Feminized Rat. The Journal of Biological Chemistry. 1990;265(15):8893–8900. [PubMed] [Google Scholar]