Abstract
The proteoglycan versican is essential to the formation of endocardial cushion mesenchyme by epithelial–mesenchymal transformation (EMT). A potentially important factor in the regulation of versican activity during cushion EMT is proteolysis by ADAMTS metalloproteinases. Using antibodies to the DPEAAE neoepitope created by ADAMTS proteolysis of versican, we detected the amino terminal 70-kDa versican cleavage fragment in cardiac cushions. Initially (i.e., 9.5 days post coitum [dpc]), the fragment is associated with endocardial cells undergoing EMT and with newly derived mesenchymal cells. ADAMTS-1 and its cofactor fibulin-1 were also associated with these cells. As cushions become increasingly populated with mesenchymal cells (10.5–12.5 dpc), the fragment remains asymmetrically distributed compared with the pattern of total versican. Highest levels of the fragment are present in regions immediately subjacent to the endocardium characterized as having densely packed, rounded cells, lacking cellular protrusions. With further development (i.e., 12.5–14.5 dpc), the pattern of fragment distribution within cushions broadens to include the ECM surrounding loosely packed mesenchymal cells in the cushion core. Together, the findings reveal that versican proteolysis leading to the production of the 70-kDa fragment is integral to the formation and differentiation of endocardial cushion mesenchyme.
Keywords: versican, epithelial–mesenchymal transformation, endocardial cushion, valvulogenesis, fibulin-1, MMP-2, ADAMTS-1, ADAMTS-9
INTRODUCTION
Endocardial cushions are expanded regions of extracellular matrix (ECM) separating the myocardium and endocardium of the early vertebrate heart tube. These structures initially become populated by mesenchymal cells through the process of epithelial–mesenchymal transformation (EMT) of the endocardium. Ultimately, endocardial cushions of the atrioventricular (AV) and conaltruncal regions give rise to cardiac valves and septa.
Versican is a member of a family of hyaluronan aggregating chondroitin sulfate proteoglycans (Yamaguchi, 2000; Wu et al., 2005), which is highly expressed during endocardial cushion formation (Henderson and Copp, 1998; Zanin et al., 1999). In mice homozygous for a transgene insertional mutation disrupting the versican gene, endocardial cushions fail to become populated by mesenchymal cells (Yamamura et al., 1997). Because versican antibodies do not react with these embryos, the versican mutant (called the heart defect [hdf] mouse), is considered a model of versican deficiency (Mjaatvedt et al., 1998; Gillotte et al., 2003; Snow et al., 2005; Williams et al., 2005). Together, these data suggest that versican is required as a positive effector of the EMT and/or for the growth of the cushion mesenchymal cells derived from the EMT. Indeed, the ability of versican to influence cellular proliferation has been established (Yang et al., 1999; Zhang et al., 1999; Wu et al., 2004, 2005; Sheng et al., 2005).
Alternative splicing of versican pre-mRNA results in transcripts that encode four variants named V0, V1, V2, and V3 (Ito et al., 1995; Zako et al., 1995). The four resulting variant core proteins have amino and carboxy terminal globular domains, designated G1 and G3. The G1 domain contains hyaluronan (HA) and link protein binding sites, whereas the G3 domain contains binding sites for beta(1)-integrins (Wu et al., 2002) and several ECM components, including fibulin-1 and −2 (Aspberg et al., 1999; Olin et al., 2001). The four variant core proteins differ in the number of glycosaminoglycan (GAG) chain binding regions with V0 containing both GAGα and GAGβ domains, V1 containing only GAGβ, V2 containing only GAGα, and V3 lacking any GAG binding domains.
In addition to alternative splicing, a potentially important dimension to the function of versican is its proteolytic cleavage by matrix metalloproteinases (MMPs; Sandy et al., 2001; Russell et al., 2003; Westling et al., 2004). Numerous MMPs, including MMP-1, -2, −3, −7, and −9, have been shown to degrade versican (Perides et al., 1995; Halpert et al., 1996; Passi et al., 1999). In addition, three members of the ADAMTS (a dis-integrin and metalloproteinase with thrombospondin motifs) family, AD-AMTS-1, −4, and −9 have also been shown to mediate versican proteolysis (Sandy et al., 2001; Somerville et al., 2003). Cleavage by these ADAMTS family members leads to production of amino-terminal fragments that can be detected using an antiserum recognizing the neoepitope sequence DPEAAE created by the cleavage (Sandy et al., 2001; Somerville et al., 2003).
Evidence for the functional significance for ADAMTS-mediated cleavage of versican is limited. The amino terminal cleavage fragments are detectable in normal adult aorta and ovary (Sandy et al., 2001; Somerville et al., 2003). Furthermore, versican cleavage by AD-AMTS-1 is required for the migration of oocytes during ovulation (Russell et al., 2003). Whether ADAMTS-mediated versican proteolysis occurs during development, particularly in the process of versican-dependent valvuloseptal morphogenesis, has not been established. On the basis of RNA in situ hybridization analysis, two of the versican cleaving ADAMTS family members, ADAMTS-1 and −9 have been reported to be expressed during early heart development (Thai and Iruela-Arispe, 2002; Jungers et al., 2005). Fibulin-1, a cofactor for ADAMTS-1 (Lee et al., 2005) and perhaps ADAMTS-9 (Hesselson et al., 2004), and a versican-binding protein is also expressed during cushion morphogenesis (Spence et al., 1992; Zhang et al., 1993, 1995; Bouchey et al., 1996). These observations raise the possibility that versican cleavage may occur in cardiac cushion morphogenesis and impact the vital activity of the proteoglycan.
RESULTS
Versican, the Versican Proteinases ADAMTS-1 and ADAMTS-9, and the ADAMTS-1 Cofactor Fibulin-1 Are Expressed During Cardiac Morphogenesis
Reverse transcriptase-polymerase chain reaction (RT-PCR) -based transcript profiling was performed on 10.5–16.5 days post coitum (dpc) embryonic mouse hearts. As shown in Figure 1, transcripts encoding the versican splice variants V0, V1, and V2 were expressed in 10.5–16.5 dpc hearts. By contrast, little or no V3 mRNA was detected in heart RNA preparations from these stages. Of the three ADAMTS proteinases known to cleave versican, transcripts encoding ADAMTS-1 and AD-AMTS-9 but not ADAMTS-4, were detectable in the 10.5–16.5 dpc developing heart. Analysis of the expression of transcripts encoding fibulin-1, an ADAMTS cofactor (Lee et al., 2005) and versican binding protein (Aspberg et al., 1999), showed that both fibulin-1C and D splice variants were expressed during heart development.
Fig. 1.
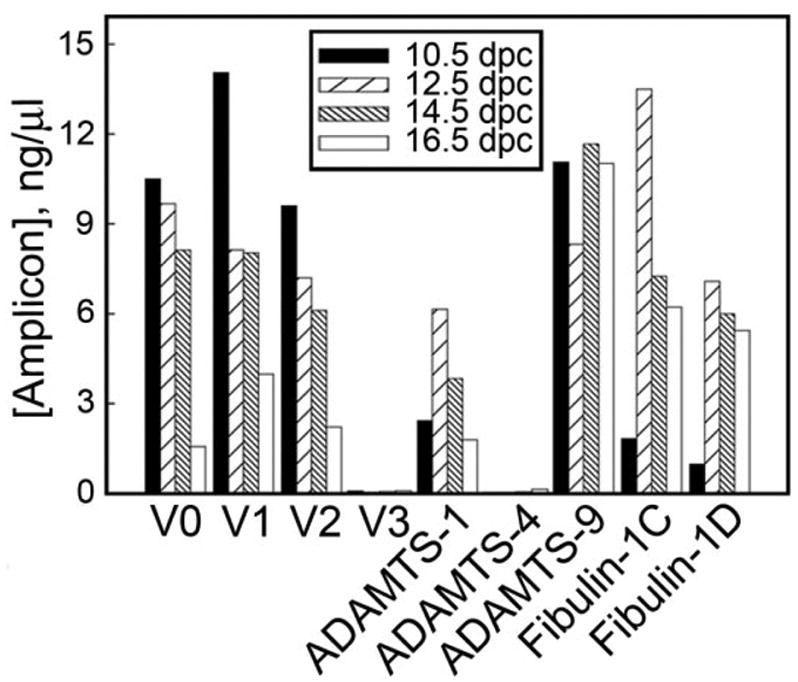
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of the expression of versican splice variants ADAMTS-1, ADAMTS-4, ADAMTS-9 and fibulin-1 splice variants during embryonic heart development. RT-PCR reactions were performed on cDNA templates made from RNA isolated from whole embryonic hearts of indicated stage. Amplicon levels were quantified using an Agilent Biosensor. Note that versican V3 and ADAMTS-4 transcripts were only slightly above the level of detection in these tissues.
Immunoblot Detection of ADAMTS-1 Isoforms in Embryonic Heart Extracts
While ADAMTS-1 mRNA expression in the embryonic heart has been reported previously (Thai and Iruela-Arispe, 2002), the expression of the protein had not been evaluated during early stages of heart development. By immunoblotting, 110-, 87-, and 65-kDa ADAMTS-1 pro and mature forms were detected in extracts from 9.5, 10.5, 12.5, 14.5, and 16.5 dpc hearts (Fig. 2). The 110-kDa immunoreactive polypeptide corresponds to the proform (open arrowhead), the 87-kDa and 65-kDA bands corresponds to the catalytically active form (filled arrowheads; Rodriguez-Manzaneque et al., 2000). These findings suggest that catalytically active forms of AD-AMTS-1 are expressed in the developing mouse heart. The lack of adequate antibodies to murine ADAMTS-9 precluded us from investigating its expression in developing heart.
Fig. 2.
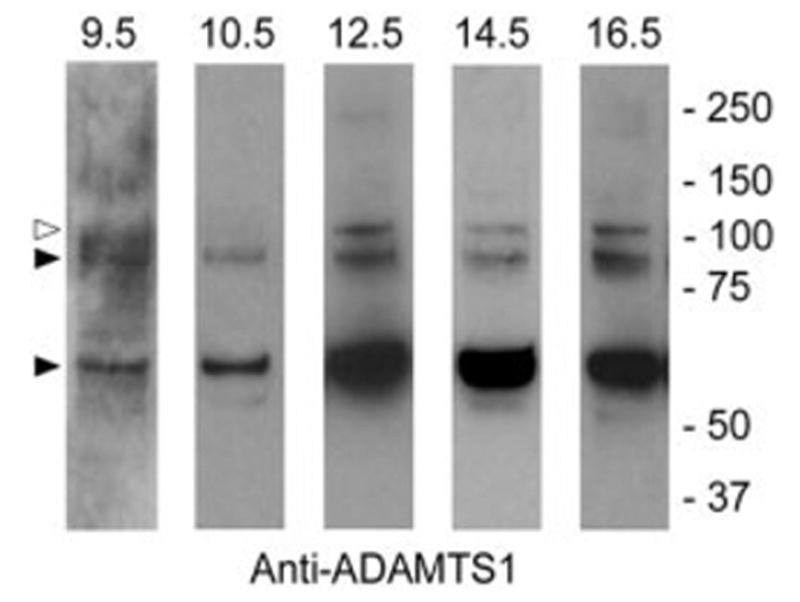
Immunoblot analysis of ADAMTS-1 in the developing mouse heart. ADAMTS-1 was detected by immunoblotting in whole embryonic heart extracts using guinea pig anti mouse ADAMTS-1 IgG. The open arrowhead points to the 110-kDa proform. Filled arrowheads point to the 87- and 65-kDa catalytically active forms. Extracts were electrophoresed under reducing conditions.
Immunoblot Detection of Versican Cleavage Products in Embryonic Heart Extracts
Proteoglycan-enriched extracts of embryonic mouse hearts were analyzed by immunoblotting using neoepitope DPEAAE reactive antiserum. This neoepitope is created by ADAMTS-1 cleavage of either versican V0 or V1 variants. As shown in Figure 3, extracts from 9.5, 10.5, 12.5, 14.5, and 16.5 dpc hearts contain an anti-DPEAAE–immunoreactive ~70-kDa fragment that corresponds in size to the product of versican V1 cleavage by ADAMTS-1 as described by Sandy et al. (2001). In some of the extracts an immunoreactive polypeptide with an apparent Mr of 37 kDa was also evident. These findings indicate that versican cleavage products, presumably generated by the action of ADAMTS metalloproteinases, are produced in the heart during stages of valvuloseptal development. The DPEAAE immunoreactive 70-kDa band was consistently detected with several different lots of DPEAAE antibody.
Fig. 3.
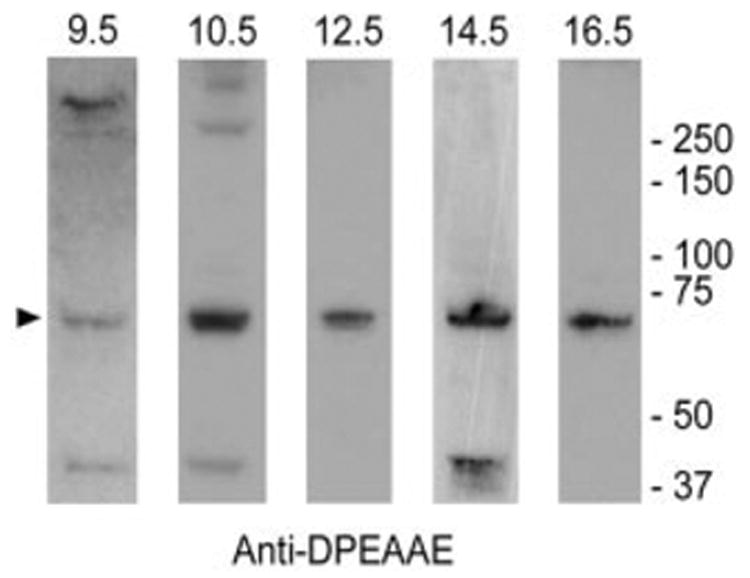
Immunoblot analysis of 70-kDa versican fragment in the developing mouse heart. Versican cleavage products were detected in embryonic heart extracts by immunoblotting using anti-DPEAAE IgG (reactive with the neoepitope generated by ADAMTS-1 cleavage of versican V1 in the GAG-β domain). Extracts were electrophoresed under reducing conditions.
Versican Cleavage Products and Proteolytic Factors Are Expressed During Early Stages of Cardiac Cushion Morphogenesis
Immunohistochemical analysis of 9.5 dpc hearts was performed to localize versican and versican cleavage products within the heart (Fig. 4). Using antibodies to the GAGβ attachment region (a domain present in versican V0 and V1), versican could be detected in the ECM of the endocardial cushions of the AV canal (AVC), the out-flow tract (OFT), the aortic sac (AS) of the anterior heart field and the ventricular subendocardium (Fig. 4A). In addition to the ECM surrounding cushion mesenchymal cells, deposits of versican could also be seen spanning acellular spaces between endocardial and myocardial layers (Fig. 4B, open arrowhead). Versican was also detected in association with endocardial cells. Little or no versican staining was evident in the myocardium.
Fig. 4.
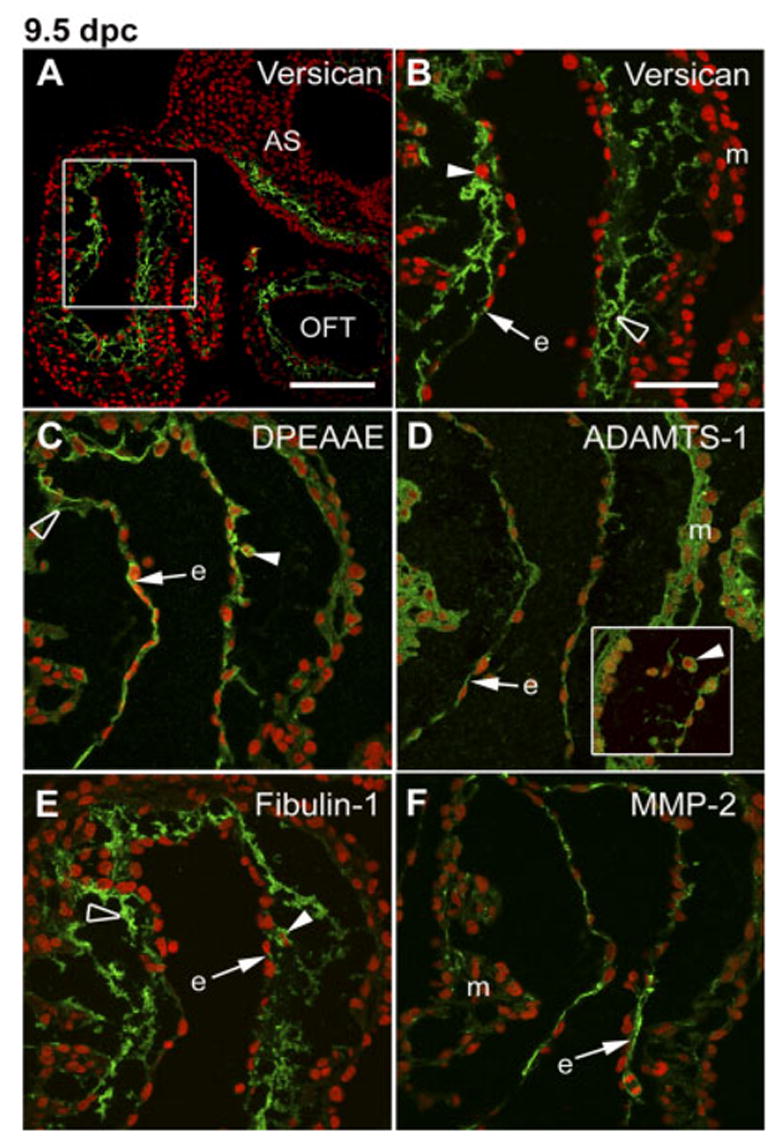
Immunolocalization of versican, its anti-DPEAAE–reactive cleavage product as well as ADAMTS-1 and fibulin-1 in the 9.5 dpc atrioventricular canal (AVC) cushion. A–F: Embryonic mouse heart sections (9.5 days post coitum [dpc]) were immunolabeled with anti-versican GAGβ IgG (A,B), anti-DPEAAE IgG (C), anti-ADAMTS-1 IgG (D), anti-fibulin-1 IgG (E), and anti–MMP-2 (F). Controls were performed using conjugated secondary IgG only (fluorescein isothiocyanate– conjugated goat anti-rabbit) showed no detectable labeling of sections of 9.5 dpc hearts. B–F: High-magnification views of the AVC. Arrowheads point to mesenchymal cells located within the AVC endocardial cushion. Open arrowhead points to immune deposits clearly located in the extracellular space between cushion mesenchymal cells. Arrows point to immunostaining associated with the endocardium. The inset panel in D shows ADAMTS-1–positive mesenchymal cells within AVC from a different section. V, ventricle; A, atrium; AA, aortic arch; OFT, outflow tract; m, myocardium; e, endocardium. Nuclei are stained red using propidium iodide. Scale bars = 150 μm in A, 50 μm in B (applies to B–F).
The DPEAAE neoepitope antiserum was used to localize the amino terminal versican cleavage product in the 9.5 dpc heart. As shown in Figure 4C, anti-DPEAAE immuno-fluorescence staining was detectable in association with endocardial cells and in the ECM of mesenchymal cells (open arrowhead) located in the endocardial cushion of the AVC. Similar staining was seen in the OFT cushion of the 9.5 heart (data not shown). Little or no anti-DPEAAE staining was evident in the myocardium at 9.5 dpc. It must be pointed out that a database search using the sequence DPEAAE found that protein phosphatase 1 (Ppp1rla), an intracellular protein, contains the sequence. Whether the antibody can cross-react with this protein is uncertain. That immunostaining is clearly evident in the extracellular spaces around mesenchymal cells within endocardial cushions at 9.5 dpc (Fig. 4C, open arrowhead) is evidence that the DPEAAE antibody is detecting the versican fragment as opposed to an intracellular protein. Additional evidence came from immunoblot analysis of total embryonic heart extracts, which showed that the DPEAAE antibody did not detect polypeptide bands with apparent Mr values corresponding to Ppp1rla or its fragments (data not shown). Moreover, a DNA microarray-based analysis of mRNAs isolated from dissected 10.5 and 11 dpc AVC cushions shows no detectable hybridization to the Ppp1rla probe pair set present on the MOE430A Affymetrix GeneChip arrays (A. Wessels and W.S. Argraves, unpublished findings).
The localization of ADAMTS-1 and fibulin-1 was also evaluated in the 9.5 dpc heart. ADAMTS-1 immunolabeling was detected in association with cells of the endocardium, myocardium (Fig. 4D), and cushion mesenchyme (inset of Fig. 4D). Fibulin-1 immunostaining was observed predominantly in the ECM of cardiac cushion mesenchymal cells, with a limited degree of staining associated with the endocardium. Little or no fibulin-1 immunolabeling was detected in the myocardium (Fig. 4E). We also evaluated the expression of MMP-2, an MMP also known to mediate versican cleavage (Passi et al., 1999). As shown in Figure 4F, MMP-2 expression was pronounced in the endocardial cells at 9.5 dpc and also evident in a limited number of cells within the myocardium.
Cushion Distribution of Versican Cleavage Products Is Asymmetric and Coincident With Compacted Cells Subjacent to Endocardium at 10.5 dpc
In the 10.5 dpc mouse heart, strong versican (anti-GAGβ reactive) immunolabeling was observed in the OFT (not shown) and AVC cushions (Fig. 5A). The pattern of versican staining within the cushions was asymmetric, with high levels observed in the ECM within the core of the cushion and low levels in regions of the cushion immediately subjacent to the endocardium (Fig. 5A). Using Nomarski differential interference contrast microscopy, the core region, an area of strong versican staining, was composed of loosely packed mesenchymal cells, whereas the region underlying the endocardium, having low levels of versican staining, was composed of densely packed, rounded cells (Fig. 5B). By contrast, anti-DPEAAE staining was not detectable in the ECM of the core region but was strong in the ECM of the layer of the densely packed cells subjacent to the endocardium (Fig. 5C,D).
Fig. 5.
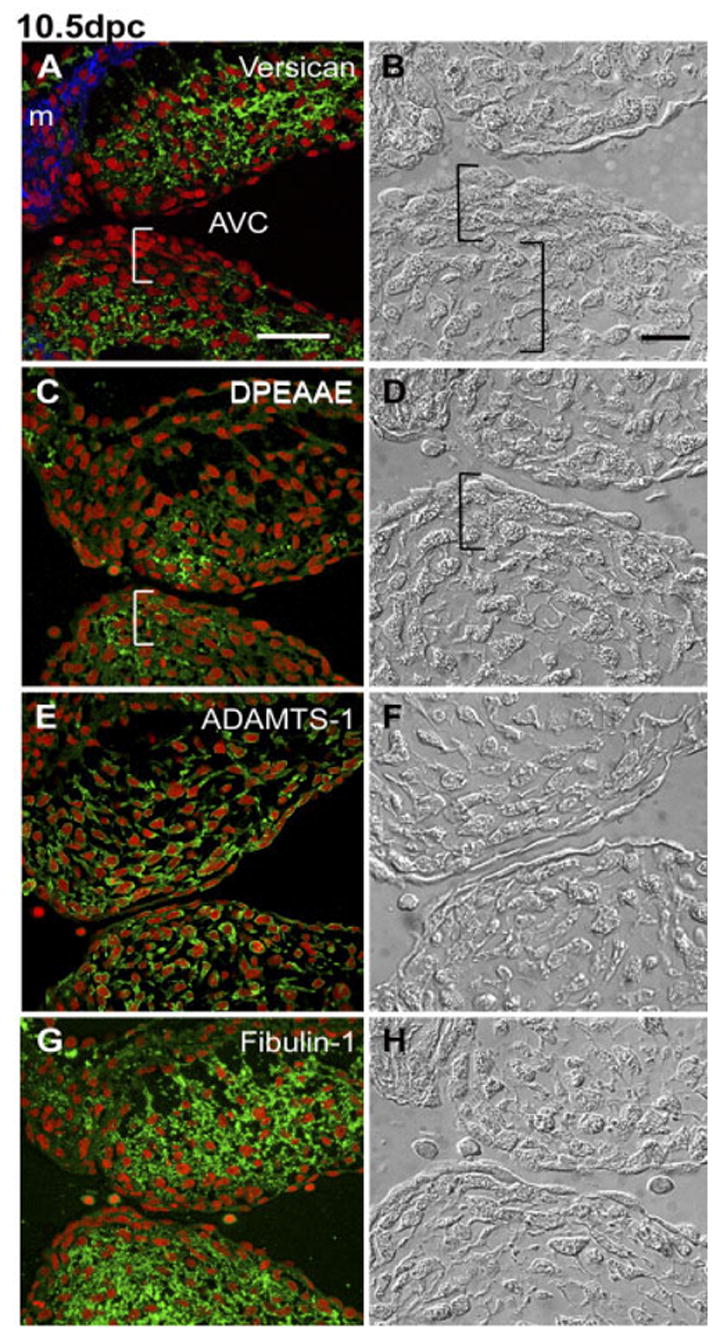
Immunolocalization of versican, its anti-DPEAAE–reactive cleavage product as well as ADAMTS-1 and fibulin-1 in the 10.5 days post coitum (dpc) atrioventricular canal (AVC) cushion. A,C,E,F: Embryonic mouse heart sections (10.5 dpc) were immunolabeled with anti-versican GAGβ IgG (A), anti-DPEAAE IgG (C), anti-ADAMTS-1 IgG (E), and anti–fibulin-1 IgG (F). B,D,F,H: Nomarski differential interference contrast images corresponding to the immunostained sections shown in A, C, E, G. Left brackets indicate compacted cells located subjacent to the endocardium. Right bracket in B indicates loosely packed mesenchymal cells. m, myocardium; AVC, AV canal. Nuclei are stained red using propidium iodide. Scale bars = 50 μm in A (applies to A,C,E,G), 20 μm in B (applies to B,D,F,H).
Anti-ADAMTS-1 immunolabeling of 10.5 dpc OFT (not shown) and AVC cushions showed that both endocardial cells and mesenchymal cells dispersed throughout the cushion expressed ADAMTS-1 (Fig. 5E). Anti–fibulin-1 staining of the 10.5 dpc OFT (not shown) AVC cushions showed high levels of immunolabeling in the ECM within the mesenchymal cushion core (Fig. 5G). By contrast, the level of fibulin-1 immunolabeling was lower in the ECM of the densely packed region associated with the endocardium (Fig. 5G,H).
Asymmetric Distribution of Versican Cleavage Products Is Maintained in the 12.5 dpc Cushion
Analysis of 12.5 dpc AVC cushions showed that anti-GAGβ–reactive versican is predominantly in the ECM of the mesenchymal core of both inferior and superior cushions (Fig. 6A,B) but not in the ECM surrounding densely packed cells (Fig. 6B, left bracket). In the inferior AVC cushion, the DPEAAE fragment was predominantly detected in the spaces between cells in the densely packed cell region with relatively lower levels of labeling in the ECM of mesenchymal cells in the cushion core (Fig. 6C,D, left bracket). By contrast, in the superior AVC cushion, relatively higher levels of anti-DPEAAE immunolabeling could be seen surrounding loosely packed mesenchymal cells in regions of cushion proximal to the endocardium (Fig. 6C, arrow). However, in deeper regions of the superior AVC cushion, lying adjacent to the myocardium, the ECM surrounding loosely packed mesenchymal cells was devoid of DPEAAE immunoreactivity but relatively rich in anti-GAGβ–reactive versican (Fig. 6C, arrowhead).
Fig. 6.
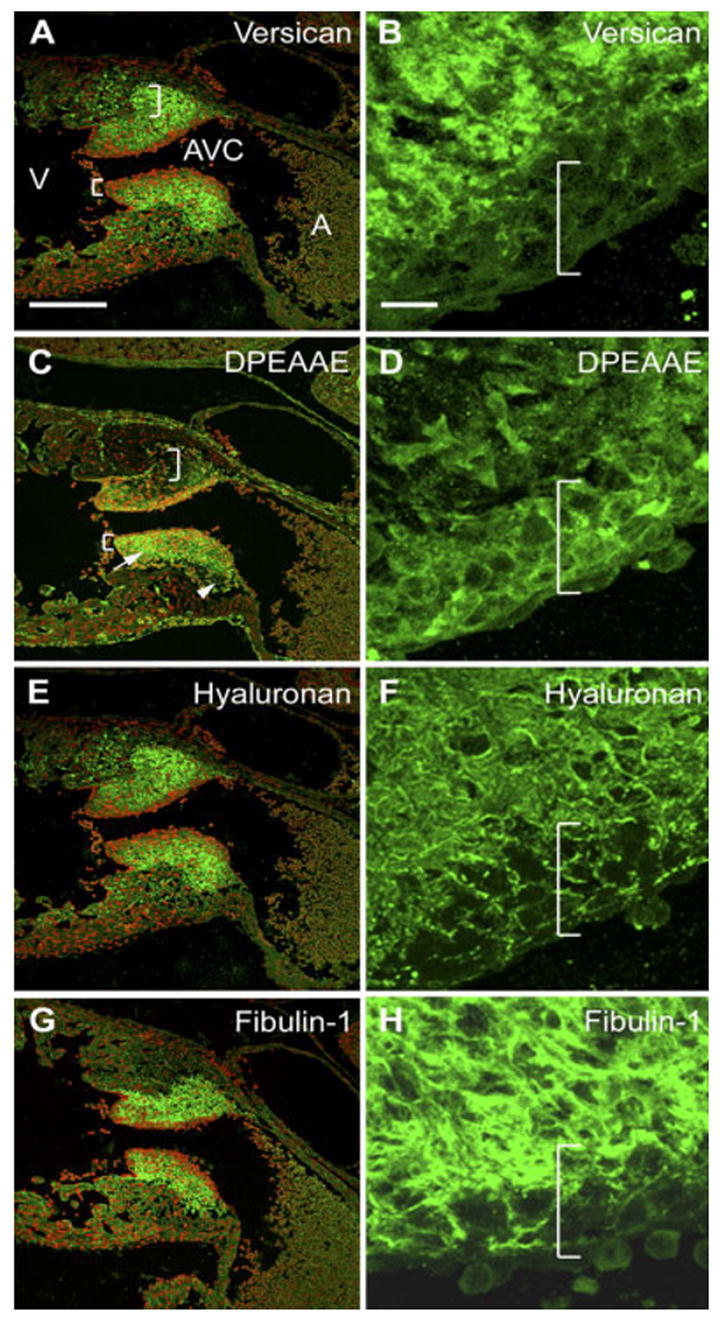
Localization of versican, its anti-DPEAAE–reactive cleavage product as well as fibulin-1 and hyaluronan in the 12.5 days post coitum (dpc) AVC cushion. A–H: Embryonic mouse heart serial sections (12.5 dpc) were immunolabeled with anti-versican GAGβ IgG (A,B), anti-DPEAAE IgG (C,D), bHABP (to detect HA; E,F), and anti–fibulin-1 IgG (G,H). A,C,E,G: Atrioventricular canal (AVC) regions. B,D,F,H: Enlargements of regions of inferior AVC cushions shown in A, C, E, and G, respectively (with the red channel turned off). Right brackets indicate regions of loosely packed mesenchyme within inferior AVC cushions. Left brackets indicate compacted cells subjacent to the endocardium. The arrow in D denotes anti-DPEAAE immunolabeling surrounding loosely packed mesenchymal cells of superior cushion that begins to appear at this stage. The arrowhead in D highlights deeper regions of the superior AVC cushion devoid of DPEAAE immunoreactivity but positive for anti-GAGβ reactive versican. V, ventricle; A, atrium. Nuclei are stained red using propidium iodide. Scale bars = 150 μm in A (applies to A,C,E,G), 15 μm in B (applies to B,D,F,H).
Fibulin-1 immunolabeling of 12.5 dpc AVC cushions shows strong staining of the ECM of the mesenchymal cushion core region as well as low-level staining within the intercellular spaces of cells in the zone of densely packed cells (Fig. 6G,H). There was no apparent difference in the distribution of fibulin-1 staining between inferior and superior AVC cushions at this stage.
Endocardial Cushion Localization of Versican or Its Amino-Terminal Fragment Are Coincident With HA Deposition
The asymmetric AVC localizations of anti-GAGβ–reactive versican and anti-DPEAAE–reactive versican raised question as to how these patterns relate to the expression of HA in the cushion ECM. Using biotinylated HABP as a probe for HA deposition, HA could be detected in both the ECM of the 12.5 dpc AVC cushion core and in the intercellular spaces between cells of the densely packed cell zone (Fig. 6E,F, left bracket). Similar results were observed in the 10.5 dpc AVC cushion (data not shown). These findings seem to rule out the possibility that an absence of HA in the densely packed cell zone is the basis for the reduced levels of anti-GAGβ–reactive versican in the zone. The data shown in Figure 6 represent adjacent sections for intact (GAGβ) or the DPEAAE versican cleaved fragment and HA. Similar results were also obtained with coimmunoflourescence of HA and either GAGβ or DPEAAE reactive antibody (data not shown). Localization of fibulin-1 in 12.5 dpc cushions was similar to that of HA, present in the cushion core ECM and in the intercellular spaces of in the densely packed cell zone This observation is consistent with fibulin-1 being indirectly associated with HA as a result of its ability to bind versican (Aspberg et al., 1999).
During Remodeling of the Cushion Levels of Versican Amino-Terminal Fragment in the Compacted Zone Are Reduced
With progressive development the AVC cushions begin to remodel to form leaflet-like structures. At 14.5 dpc, primitive AV valve leaflets have on their atrial face a region of highly compacted cells (Fig. 7C,F,I, left bracket). At this stage, the primitive AV valve leaflets have elongated, extending toward the ventricle. Immunohistological analysis of the AVC leaflets reveals that the highly compacted cell region is largely devoid of both anti-GAGβ–reactive versican and anti-DPEAAE–reactive versican (Fig. 7). In regions of loosely packed mesenchymal cells adjacent to the densely packed cells, there is relatively strong anti-GAGβ–reactive versican (Fig. 7B, right bracket) but little or no anti-DPEAAE–reactive versican (Fig. 7E, right bracket). In general, anti-GAGβ–reactive versican can be seen throughout the mesenchymal core of the primitive AV leaflets extending into the elongated portion of the leaflets. Anti-DPEAAE–reactive versican can be seen in the ECM of mesenchymal cells within the elongated portion of the leaflets. A high level of anti-DPEAAE–reactive versican underlies the endocardium of the distal portion of AV leaflets (Fig. 7E, arrow). By contrast to anti-GAGβ–reactive versican, fibulin-1 immunostaining is asymmetrically distributed in the primitive 14.5 dpc AV leaflets, localized predominantly in the ECM of mesenchymal cells within the elongated portion of the leaflets (Fig. 7G,H). Therefore, at 14.5 dpc, the proteolytic processing of versican broadens with respect to cellular arrangement and in comparison to GAGβ staining. Even though the immunostaining of DPEAAE overlaps with more regions of GAGβ immunolocalization than at earlier stages, there are still regions of loose mesenchyme in the cushion core that are devoid of DPEAAE immunostaining (Fig. 7E, right bracket).
Fig. 7.
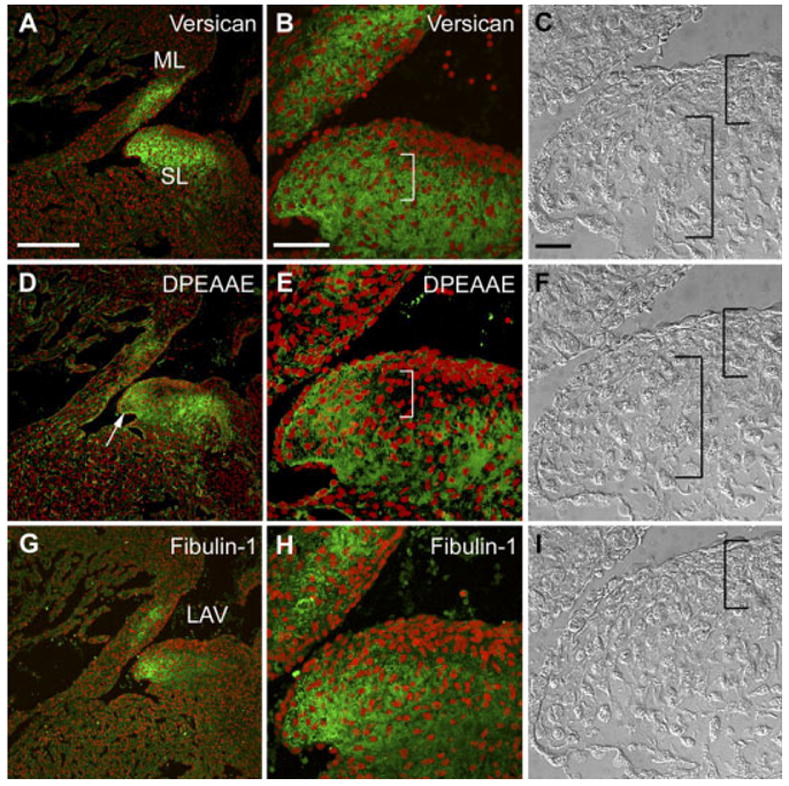
Localization of versican, its anti-DPEAAE–reactive cleavage product as well as fibulin-1 in the 14.5 days post coitum (dpc) atrioventricular canal (AVC) cushion. A,B,D,E,G,H: Embryonic mouse heart sections (14.5 dpc) were immunolabeled with anti-versican GAGβ IgG (A,B), anti-DPEAAE IgG (D,E) and anti-fibulin-1 IgG (G,H). B,E,H: Enlargements of regions of AVC cushions shown in A, D, and G, respectively. C,F,I: Nomarski differential interference contrast images corresponding to the immunostained sections shown in B, E, and H, respectively. Right brackets indicate regions of loosely packed mesenchyme within AVC cushions. Left brackets indicate compacted cells subjacent to the endocardium. ML, mural leaflet of the left AV region (LAV). SL, septal leaflet of the LAV. Arrow in E shows staining of the DPEAAE antibody subjacent to the endocardium of the elongating leaflet. Scale bars = 150 μm in A (applies to A,D,G), 50 μm in B (applies to B,E,H), 20 μm in C (applies to C,F,I).
DISCUSSION
Findings presented herein indicate that proteolytic cleavage of versican is occurring throughout the process of cardiac cushion morphogenesis. At the earliest stages of cushion morphogenesis (i.e., 9.5 dpc), immunohistological analysis shows that anti-GAGβ–reactive versican is deposited throughout the acellular portion of the cushion along with fibulin-1. By contrast, anti-DPEAAE–reactive versican is seen only in the endocardium and in association with newly formed mesenchymal cells at this stage. Both types of cells also express ADAMTS-1. Taken together, it is reasonable to conclude that versican cleavage occurring at 9.5 dpc is mediated by endocardial cells and emergent mesenchymal cells, possibly by means of the action of ADAMTS-1. Because the endocardium at this stage is undergoing EMT, the findings also implicate a role for ADAMTS-mediated versican proteolysis in the EMT process.
As cardiac EMT progresses (i.e., 9.5–10.5 dpc) and cushions become increasingly populated with mesenchymal cells, an asymmetrical pattern of anti-DPEAAE–reactive fragment deposition is observed. The fragment can be found predominantly within regions of the cushion immediately subjacent to the endocardium. These regions are characterized as having densely packed, rounded cells, lacking cellular protrusions. We speculate that the cells in these regions represent an expanding population derived from the initial events of the EMT, and thus, we refer to the regions as EMT zones. Whether cells in EMT zones retain some endothelial characteristics and are intermediates in the process of mesenchymal differentiation is uncertain. Mesenchymal cells located in close proximity to the endocardium have been shown to express N-CAM, an epithelial adhesion molecule, highly expressed by endocardial cells (Mjaatvedt and Markwald, 1989). Cells of EMT regions of 10.5–12.5 dpc cushions are morphologically distinct from the mesenchymal cells within the core of the cushion, which are loosely packed and have extensive cellular protrusions. On the basis of our immunohistological data, the ECM surrounding the loosely packed cushion mesenchymal cells within core regions contain relatively low levels of the anti-DPEAAE–reactive cleavage product but relatively high levels of anti-GAGβ–reactive versican. This finding suggests that cushion cores contain intact V0/V1 forms of versican. Together our findings not only reveal “tissue heterogeneity” within cardiac cushions but also provide evidence for a distinctive molecular composition for cushion tissue ECM, resulting from proteolytic processing of versican by the MMPs AD-AMTS-1, ADAMTS-9, and MMP-2.
The asymmetrical pattern of anti-DPEAAE–reactive versican deposition, seen within 9.5–10.5 dpc cushions, was less apparent at 12.5 dpc and was greatly reduced by 14.5 dpc. Indeed by 14.5 dpc, pronounced anti-DPEAAE staining was seen in the ECM of regions of loosely packed mesenchymal cells within the AVC cushions, although there were some regions of cushion mesenchyme still having little or no anti-DPEAAE staining. This developmental transition may highlight an expanding role for versican proteolysis in regions of the cushion beyond the EMT zone as cushions transform to mature leaflets and septal structures. A similar transition has been described for the pattern of Wnt/β-catenin signaling within the developing cardiac cushion. At early stages (i.e., 11.5 dpc), Wnt/β-catenin signaling is restricted to regions of AVC cushion adjacent to the endocardium, but by 12.5 dpc, the pattern of signaling broadens to include a larger proportion of mesenchyme (Gitler et al., 2003).
Our findings raise questions as to the significance of the cleavage to the biological activity of versican. If versican functions to promote cushion EMT, as suggested by the hdf phenotype, then proteolytic cleavage may inactivate the molecule and, thus, serve a braking function. Conversely, the EMT-promoting activity of versican may result from the proteolytic cleavage of versican producing fragment(s) having activities distinct from the intact proteoglycan. In fact, there is extensive literature on the biological activities associated with various domains of versican, including studies showing that the G1 domain reduces cell adhesion and promotes proliferation (Ang et al., 1999; Yang et al., 1999; Zhang et al., 1999). Recent findings also demonstrate that the G1 domain augments anchorage-independent growth by reducing apoptosis, resulting in a shift in the equilibrium of cells toward proliferation (Cattaruzza et al., 2004). These findings are consistent with the possibility that the 70-kDa fragment, liberated as a result of cleavage by ADAMTS-1 and fibulin-1, might act to reduce cell adhesion and promote proliferation of cardiac cushion cells. This possibility coupled with findings that anti-DPEAAE immunoreactivity is most concentrated in the densely packed cell zone underlying the 10.5–12.5 dpc endocardium may indicate that the 70-kDa fragment of versican is acting to promote proliferation and cell rounding within the zone. By contrast, differentiation might be favored over proliferation in regions of cushions in which the intact form of versican may be more prevalent than the cleaved form (i.e., in regions of loosely packed mesenchymal cells).
Although our studies implicate a role for ADAMTS proteases in the process of versican cleavage during cushion morphogenesis, the findings do not preclude the possibility that the DPEAAE epitope-containing fragment of versican might be generated by other MMPs. MMP-1, −2, −3, −7, and −9 have been shown to degrade versican (Perides et al., 1995; Halpert et al., 1996; Passi et al., 1999), but their ability to generate the DPEAAE fragment has not been established. Furthermore, the repertoire of MMPs expressed in developing cardiac cushions remains poorly resolved. MMP-2 has also been detected in avian cardiac cushions associated with mesenchymal cells immediately subjacent to the endocardium (Cai et al., 2000). In 9.5 dpc mouse hearts, we detected pronounced MMP-2 expression in AVC and OFT endocardial cells (Fig. 4F). Studies by Song et al. (2000) show that MMP-2 activity is required for in vitro endocardial cushion cell migration. MMP-2 has also been directly implicated in other EMTs, including those associated with the formation of neural crest cells and certain cancers (Kang and Svoboda, 2005; Ahmed et al., 2006; Taki et al., 2006). These findings highlight the possibility that MMP-2 may mediate proteolysis of versican during cushion EMT.
Several pieces of evidence indicate that the vital role of versican in cardiac morphogenesis is mediated through its ability to influence HA signaling by means of ErbB receptor tyrosine kinases (RTK) activation (Camenisch et al., 2002). For example, embryos deficient in either versican, the HA synthesizing enzyme Has2, or the RTK ErbB2 and ErbB3, all exhibit absence or reduction in endocardial cushion mesenchyme (Erickson et al., 1997; Mjaatvedt et al., 1998; Camenisch et al., 2000). Furthermore, treatment of Has2-deficient AVC cushion explants with the ErbB3 ligand, heregulin rescues the capacity of the mutant cushions to form mesenchyme (Camenisch et al., 2002). That heregulin treatment of the explants also restores ErbB2 phosphorylation suggests that the process of cushion cellularization is dependent on ErbB2/ErbB3 heterodimer signaling (Camenisch et al., 2002). Signaling by another ErbB receptor, epidermal growth factor receptor (EGFR, also known as ErbB1) has also been implicated in versican-mediated endocardial cushion cellularization. It has been observed that expression of full-length versican V1 acts to up-regulate the level of activated ErbB1 (Wu et al., 2004). These findings raise the possibility that the ADAMTS-mediated cleavage of versican occurring in the EMT zone might be part of a mechanism to suppress ErbB1 activity. Ample evidence indicates the important role that ErbB1 signaling plays in the control of endocardial cushion cellularization. For example, mice homozygous for a hypomorphic ErbB1 mutation develop hyperplastic semilunar valves possibly due to increased proliferation of mesenchymal cells within the cushion (Chen et al., 2000). Similarly, mice deficient in the ErbB1 ligand HB-EGF also display hyperplastic cushions (Jackson et al., 2003). These findings indicate that suppression of ErbB1 signaling leads to cushion and valvular hypercellularization. Whether such hypercellularization results from increased EMT, growth, migration, or a combination of these is not known, but it follows that, if intact versican V1 promotes ErbB1 signaling, then proteolytic cleavage could act to influence ErbB1 signaling and thereby regulate endocardial cushion cellularization.
EXPERIMENTAL PROCEDURES
Antibodies
Rabbit polyclonal antibody raised against the GAGβ domain of mouse versican was purchased from Chemicon (Temecula, CA). Rabbit polyclonal antibody against the C-terminal sequence of the fragment of versican V0/V1 generated as a result of proteolytic cleavage (anti-DPEAAE) as described by Sandy et al. (2001) was purchased from Affinity BioReagents (Golden, CO). Rabbit anti-fibulin (rb1323) has been described previously (Argraves et al., 1990). Guinea pig anti-mouse ADAMTS-1 (GP-12) has been described recently (Lee et al., 2005). Rabbit anti-mouse ADAMTS-1 metalloprotease domain (residues 317–335) was obtained from Dr. JoAnne Richards (Baylor College of Medicine; Russell et al., 2003). Mouse monoclonal anti-alpha sarcomeric actin (clone 5C5 A2172) was obtained from Sigma Chemical Co. (St. Louis, MO). Rabbit anti-human MMP-2 (collagenase IV or gelatinase A) polyclonal antibody was purchased from Chemicon International.
Immunoblotting
The amino terminal versican fragment was detected in embryonic heart extracts using a neoepitope antiserum to the C-terminal peptide sequence DPEAAE according to the procedure of Sandy et al. (2001). Briefly, equal amounts of total protein in guanidine extracts (4 M guanidine-HCl, 10 mM MES, 50 mM sodium acetate, 5 mM ethylenediaminetetraacetic acid [EDTA], containing the protease inhibitor cocktail complete Mini EDTA-free from Roche, Mannheim, Germany) of freshly harvested embryonic hearts were precipitated with ethanol. The number of embryonic hearts extracted for each stage was 25 for 9.5 dpc, 20–30 for 10.5 dpc, 10–15 for 12.5 dpc, 10 for 14.5 dpc, and 5 for 16.5 dpc. The precipitated proteins were then deglycosylated by digestion for 1.5 hr at 37°C with protease-free chondroitinase ABC (250 milli-units/400–500 μg total protein, Sigma). ADAMTS-1 was detected in detergent extracts (1% NP-40, 0.25% SDS, 150 mM NaCl, 1 mM EDTA, 50 mM Tris HCL pH 7.4, containing the Roche protease inhibitor cocktail) of whole embryonic hearts by immunoblotting. The number of embryonic hearts extracted for each stage was 20 for 9.5 dpc, 12 for 10.5 dpc, 12 for 12.5 dpc, 14 for 14.5 dpc, and 7 for 16.5 dpc.
RT-PCR
Total RNA was isolated from embryonic hearts using Trizol reagent (Invitrogen, Carlsbad, CA) and the RNeasy Minikit (Qiagen, Inc., Valencia, CA). The number of embryonic hearts extracted for each stage was 9 for 10.5 dpc, 12 for 12.5 dpc, 4 for 14.5 dpc, and 2 for 16.5 dpc. Total RNA (1 μg) was converted to cDNA using random hexamer primers and the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Primer sequences for the RT-PCR analysis were designed using the Primer3 program (Whitehead Institute for Biomedical Research, Cambridge, MA; Rozen and Skaletsky, 2000). Versican variant primers were based on mouse versican cDNA sequence accession numbers XM_488510, D16263, D28599, and D32040 for V0, V1, V2, and V3 variants, respectively. Versican V0 variant primers were (Forward) 5′-GCCAACAAGACCATCAGT-TG-3′ and (Reverse) 5′-GTAACGAGC-TGCTTCCCATT-3′. Versican V1 variant primers were (Forward) 5′-TTCAG-GCAGCTTGGAGAAAT-3′ and (Reverse) 5′-GTAACGAGCTGCTTCCC-ATT-3′. Versican V2 variant primers were (Forward) 5′-TTCAGGCAGCTT-GGAGAAAT-3′ and (Reverse) 5′-CG-GTGGCTAATGGAATGACT-3′. Versican V3 variant primers were (Forward) 5′-TTCAGGCAGCTTGGAGAAAT-3′ and (Reverse) 5′-GTGCGTCGATGAG-CAAAGTA-3′. ADAMTS-1 primers were (Forward) 5′-CAGTACCAGAC-CTTGTGCAGACCTT-3′ and (Reverse) 5′-CACACCTCACTGCTTACTGGTT-TGA-3′ and were based on mouse cDNA sequence accession number NM009621. ADAMTS-4 primers were (Forward) 5′-GAGCAGTGTGCTGCCTACAA-3′ and (Reverse) 5′-TGCTGCCGTACAAGGA-TATG-3′ and were based on mouse cDNA sequence accession number NM172845. ADAMTS-9 primers were (Forward) 5′- CAGCGTTGATGGGGA-GTATT-3′ and (Reverse) 5′-GAAACTG-GAAAAGCCAGCAG-3′ and were based on mouse cDNA sequence accession number BC068142. Fibulin-1C primers were 5′-CCCAATGGCCGCAACTGC-CAAGACATTG-3′ and (Reverse) 5′-GCCCTCCTCATTGCCAGCGGTGAT-GGC-3′ and were based on mouse fibulin-1 cDNA sequence accession number X70853. Fibulin-1D primers were (Forward) 5′-GGAGTCTCGAAGGTTC-CCTTCTGTGATG-3′ and (Reverse) 5′-GCCCTCCTCATTGCCAGC- GGTGA-TGGC-3′ and were based on mouse fibulin-1D cDNA sequence accession number X70854.
Immunohistochemistry
Normal mouse embryos (CD1 strain) at different stages of development were fixed in 2 or 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm thickness. Deparaffinized sections were rehydrated through a graded series of ethanols to phosphate buffered saline (PBS). Sections for immunostaining of versican and fibulin-1 were subjected to antigen unmasking based on a high-temperature citric acid formula (H-3300, Vector Laboratories, Burlingame, CA). Sections for ADAMTS-1 and MMP-2 immunostaining were treated with 0.1 mg/ml proteinase K. Next, sections were blocked 1 hr at room temperature with blocking buffer (PBS, Sigma containing 3% normal goat serum (NGS, Cappel, Malvern, PA), and 1% bovine serum albumin (catalog no. B4287, Sigma) then incubated overnight at 4°C with primary antibody diluted in blocking buffer (anti-DPEAAE was diluted to 0.20 μg/ml; anti-GAGβ was diluted to 5 μg/ml; fibulin-1 antibody was diluted to 50 μg/ml). After primary antibody incubations, specimens were washed 5 times in PBS and incubated at room temperature with fluorochrome-conjugated secondary antibody (Jackson Laboratories, Bar Harbor, ME) diluted in blocking buffer. All samples were washed extensively in PBS and cover-slipped using DABCO mounting media (Sigma). Nuclei were labeled with 1 μg/ml propidium iodide (Molecular Probes C-7590) in PBS for 5 min before the final washes in PBS. Controls for immunohistochemical staining experiments included the use of the secondary antibody only and use of pre-immune serum. Immunostained sections were analyzed using a Leica TCS SP2 AOBS Confocal Microscope System (Leica Microsystems, Inc., Exton, PA).
Detection of Hyaluronan in Embryonic Tissue Sections
Hyaluronan was detected in embryonic heart tissue sections using a modification of the technique of Toole et al. (2001). Briefly, tissues sections were incubated with 2 μg/ml biotinylated hyaluronic acid binding protein (bHABP, Seikagaku America, East Falmouth, MA; a complex of aggrecan HA binding domain and link protein) in 10% fetal calf serum for 1 hr at room temperature. To detect bound bHABP, sections were incubated for 1 hr in fluorescein avidin DCS (at 20 μg/ml; Vector Laboratories, Burlington, CA) in 0.1 M sodium bicarbonate buffer containing 0.85% sodium chloride and evaluated by confocal microscopy. Control experiments included incubation of tissue sections with 6 units/ml streptomyces hyaluronidase for 1 hr at 37°C before addition of bHABP and fluorescein avidin DCS.
Footnotes
Grant sponsor: NIH; Grant numbers: HL52813; HL61873; HL66231; CA73837; CA82867; CA77420; CA65624.
References
- Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating epidermal growth factor-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00478.2005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ang LC, Zhang Y, Cao L, Yang BL, Young B, Kiani C, Lee V, Allan K, Yang BB. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J Neuropathol Exp Neurol. 1999;58:597–605. doi: 10.1097/00005072-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Argraves WS, Tran H, Burgess WH, Dickerson K. Fibulin is an extracellular matrix and plasma glycoprotein with repeated domain structure. J Cell Biol. 1990;111:3155–3164. doi: 10.1083/jcb.111.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspberg A, Adam S, Kostka G, Timpl R, Heinegard D. Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. J Biol Chem. 1999;274:20444 –20449. doi: 10.1074/jbc.274.29.20444. [DOI] [PubMed] [Google Scholar]
- Bouchey D, Argraves WS, Little CD. Fibulin-1, vitronectin, and fibronectin expression during avian cardiac valve and septa development. Anat Rec. 1996;244:540 –551. doi: 10.1002/(SICI)1097-0185(199604)244:4<540::AID-AR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cai DH, Vollberg TM, Sr, Hahn-Dantona E, Quigley JP, Brauer PR. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat Rec. 2000;259:168 –179. doi: 10.1002/(SICI)1097-0185(20000601)259:2<168::AID-AR7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349 –360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850 –855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Cattaruzza S, Schiappacassi M, Kimata K, Colombatti A, Perris R. The globular domains of PG-M/versican modulate the proliferation-apoptosis equilibrium and invasive capabilities of tumor cells. FASEB J. 2004;18:779 –781. doi: 10.1096/fj.03-0660fje. [DOI] [PubMed] [Google Scholar]
- Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24:296 –299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- Erickson SL, O’Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999 –5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Gillotte DM, Fox PL, Mjaatvedt CH, Hoffman S, Capehart AA. An in vitro method for analysis of chondrogenesis in limb mesenchyme from individual transgenic (hdf) embryos. Methods Cell Sci. 2003;25:97–104. doi: 10.1007/s11022-004-9803-3. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Jiang YQ, Epstein JA, Gruber PJ. Molecular markers of cardiac endocardial cushion development. Dev Dyn. 2003;228:643–650. doi: 10.1002/dvdy.10418. [DOI] [PubMed] [Google Scholar]
- Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748 –9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ Res. 1998;83:523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- Hesselson D, Newman C, Kim KW, Kimble J. GON-1 and fibulin have antagonistic roles in control of organ shape. Curr Biol. 2004;14:2005–2010. doi: 10.1016/j.cub.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Ito K, Shinomura T, Zako M, Ujita M, Kimata K. Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J Biol Chem. 1995;270:958 –965. doi: 10.1074/jbc.270.2.958. [DOI] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704 –2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungers KA, Le Goff C, Somerville RP, Apte SS. Adamts9 is widely expressed during mouse embryo development. Gene Expr Patterns. 2005;5:609 –617. doi: 10.1016/j.modgep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kang P, Svoboda KK. Epithelial-mesenchymal transformation during craniofacial development. J Dent Res. 2005;84:678 –690. doi: 10.1177/154405910508400801. [DOI] [PubMed] [Google Scholar]
- Lee NV, Rodriguez-Manzaneque JC, Thai SN, Twal WO, Luque A, Lyons KM, Argraves WS, Iruela-Arispe ML. Fibulin-1 acts as a cofactor for the matrix metalloprotease ADAMTS-1. J Biol Chem. 2005;280:34796 –34804. doi: 10.1074/jbc.M506980200. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Markwald RR. Induction of an epithelial-mesenchymal transition by an in vivo adheron-like complex. Dev Biol. 1989;136:118 –128. doi: 10.1016/0012-1606(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56 –66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Olin AI, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. The proteoglycans aggrecan and Versican form networks with fibulin-2 through their lectin domain binding. J Biol Chem. 2001;276:1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- Passi A, Negrini D, Albertini R, Miserocchi G, De Luca G. The sensitivity of versican from rabbit lung to gelatinase A (MMP-2) and B (MMP-9) and its involvement in the development of hydraulic lung edema. FEBS Lett. 1999;456:93–96. doi: 10.1016/s0014-5793(99)00929-1. [DOI] [PubMed] [Google Scholar]
- Perides G, Asher RA, Lark MW, Lane WS, Robinson RA, Bignami A. Glial hyaluronate-binding protein: a product of metalloproteinase digestion of versican? Biochem J. 1995;312:377–384. doi: 10.1042/bj3120377. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Milchanowski AB, Dufour EK, Leduc R, Iruela-Arispe ML. Characterization of METH-1/ADAMTS1 processing reveals two distinctactiveforms. JBiolChem. 2000;275:33471–33479. doi: 10.1074/jbc.M002599200. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologistprogrammers. MethodsMolBiol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330 –42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, Wu Y, Lee V, Slingerland J, Dumont D, Yang BB. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330 –1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow HE, Riccio LM, Mjaatvedt CH, Hoffman S, Capehart AA. Versican expression during skeletal/joint morphogenesis and patterning of muscle and nerve in the embryonic mouse limb. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:95–105. doi: 10.1002/ar.a.20151. [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Song W, Jackson K, McGuire PG. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev Biol. 2000;227:606 –617. doi: 10.1006/dbio.2000.9919. [DOI] [PubMed] [Google Scholar]
- Spence SG, Argraves WS, Walters L, Hungerford JE, Little CD. Fibulin is localized at sites of epithelial-mesenchymal transitions in the early avian embryo. Dev Biol. 1992;151:473–484. doi: 10.1016/0012-1606(92)90186-k. [DOI] [PubMed] [Google Scholar]
- Taki M, Verschueren K, Yokoyama K, Nagayama M, Kamata N. Involvement of Ets-1 transcription factor in inducing matrix metalloproteinase-2 expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Int J Oncol. 2006;28:487–496. [PubMed] [Google Scholar]
- Thai SN, Iruela-Arispe ML. Expression of ADAMTS1 during murine development. Mech Dev. 2002;115:181–185. doi: 10.1016/s0925-4773(02)00115-6. [DOI] [PubMed] [Google Scholar]
- Toole BP, Yu Q, Underhill CB. Hyaluronan and hyaluronan-binding proteins. Probes for specific detection. Methods Mol Biol. 2001;171:479 –485. doi: 10.1385/1-59259-209-0:479. [DOI] [PubMed] [Google Scholar]
- Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD. ADAMTS4 (aggre-canase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J. 2004;377:787–795. doi: 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Jr, Presar AR, Richmond AT, Mjaatvedt CH, Hoffman S, Capehart AA. Limb chondrogenesis is compromised in the versican deficient hdf mouse. Biochem Biophys Res Commun. 2005;334:960 –966. doi: 10.1016/j.bbrc.2005.06.189. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen L, Zheng PS, Yang BB. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277:12294 –12301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- Wu Y, Sheng W, Chen L, Dong H, Lee V, Lu F, Wong CS, Lu WY, Yang BB. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol Biol Cell. 2004;15:2093–2104. doi: 10.1091/mbc.E03-09-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276 –289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186:58 –72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- Yang BL, Zhang Y, Cao L, Yang BB. Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem. 1999;72:210 –220. doi: 10.1002/(sici)1097-4644(19990201)72:2<210::aid-jcb5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Zako M, Shinomura T, Ujita M, Ito K, Kimata K. Expression of PG-M(V3), an alternatively spliced form of PG-M without a chondroitin sulfate attachment in region in mouse and human tissues. J Biol Chem. 1995;270:3914 –3918. doi: 10.1074/jbc.270.8.3914. [DOI] [PubMed] [Google Scholar]
- Zanin MK, Bundy J, Ernst H, Wessels A, Conway SJ, Hoffman S. Distinct spatial and temporal distributions of aggrecan and versican in the embryonic chick heart. Anat Rec. 1999;256:366 –380. doi: 10.1002/(SICI)1097-0185(19991201)256:4<366::AID-AR4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Kluge M, Timpl R, Chu ML, Ekblom P. The extracellular matrix glycoproteins BM-90 and tenascin are expressed in the mesenchyme at sites of endothelial-mesenchymal conversion in the embryonic mouse heart. Differentiation. 1993;52:211–220. doi: 10.1111/j.1432-0436.1993.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Chu ML, Pan TC, Sasaki T, Timpl R, Ekblom P. Extracellular matrix protein fibulin-2 is expressed in the embryonic endocardial cushion tissue and is a prominent component of valves in adult heart. Dev Biol. 1995;167:18 –26. doi: 10.1006/dbio.1995.1003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Kiani C, Yang BL, Hu W, Yang BB. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem. 1999;73:445–457. [PubMed] [Google Scholar]


