Abstract
The diterpene glycoside stevioside (SVS) and soy bean protein isolate have both been shown to have beneficial effects in diabetes treatment. As they each show different benefits we investigated whether the combination of both substances shows an improvement in the treatment of diabetes in Goto-Kakizaki (GK) rats. Over the course of 4 wk, the rats were fed with the following four test diets (n = 12 per group): 1. Standard carbohydrate-rich laboratory diet (chow), 2. chow + SVS (0.03 g/kg BW/day), 3. 80% SPI + 20% chow and 4. 80% SPI + 20 % chow + SVS (0.03 g/kg BW/day). At the end of the course conscious rats underwent an intra-arterial glucose tolerance test (IAGTT) (2.0 g glucose/kg BW). Compared to normal chow diet, stevioside in combination with SPI shows the following beneficial effects in GK rats with mild type 2 diabetes: 1. a 56% reduction in plasma glucose (p < 0.001), 2. a 118% increase in first-phase insulin (p < 0.005), 3. a 20% reduction in glucagons (p < 0.05), 4. a 28% reduction in total cholesterol (p < 0.001), 5. a 13% reduction in FFA (p < 0.01), 6. a 49% reduction in TG (p < 0.001) and 7. a 11% reduction in the systolic blood pressure (p < 0.001). In conclusion, the combination of stevioside and SPI has synergistic positive effects on the characteristic features of the metabolic syndrome, i.e. hyperglycemia, hypertension and dyslipidemia.
Keywords: type 2 diabetes mellitus, metabolic syndrome, stevioside, soy-protein isolate, GK-rats
Introduction
Type 2 diabetes mellitus and the metabolic syndrome have become major public health concerns in industrialized and developing countries [1]. This is due to their increasing prevalence in epidemic proportions, and their frequent association with major cardiovascular risk factors and cardiovascular diseases. Type 2 diabetes is associated with a 4-6 fold increase in mortality caused by coronary artery disease (CAD) [2]. Approximately 50% of type 2 diabetic subjects suffer from hypertension and dyslipidemia and a 2-3 fold increased CAD risk is seen at any cholesterol level [3].
Type 2 diabetes mellitus is characterized by impaired insulin secretion and peripheral insulin resistance [4]. The treatment of type 2 diabetes is currently unsatisfactory. Therefore, new agents are needed. The first aim is to develop drugs that augment insulin secretion through a mechanism other than blocking K+ATP-channels. The second aim is to provide cheap drugs for the growing population of diabetes sufferers in developing countries which cannot afford medicines currently available from industrialized countries. Traditional anti-diabetic plants might provide a useful source of new oral anti-hyperglycemic compounds, or supply simple dietary adjuncts to existing therapies. On this account, the World Health Organization Expert Committee on Diabetes has recommended investigation into traditional methods for the treatment of diabetes [5, 6]. As a traditional medicine, the plant Stevia rebaudiana Bertoni (SrB) is used in the treatment of diabetes among the Guarani Indians in Paraguay and Brazil [7, 8]. Oral intake of SrB extracts has been shown to suppress plasma glucose in diabetic subjects [9, 10]. The diterpene glycoside, stevioside (19-O-β-glucopyranosyl-13-O-β-glucopyranosyl (1-2)-β-glucopyranosyl-steviol) (Figure 1), isolated from SrB, exhibits a direct insulinotropic action in both isolated mouse islets and the clonal beta-cell lines (INS-1) [11, 12]. Furthermore, we have shown that stevioside possesses insulinotropic, glucagonostatic, anti-hyperglycemic and blood pressure-lowering effects in diabetic animals [13, 14]. Stevioside also exerts an anti-hyperglycemic action on postprandial blood glucose levels in type 2 diabetic subjects [15].
Figure 1.
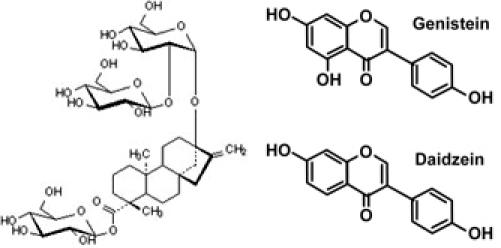
Structures of stevioside and isoflavones (daidzein and genistein).
Soy intake has been linked to improved blood lipid levels and decreased arterial fatty streaks, thereby reducing the risk of atherosclerosis [2, 16-19]. The soy components that may exert beneficial cardiovascular effects include protein, lipids, fiber and phytochemicals, including isoflavones. Soy proteins have been shown to decrease serum cholesterol, LDL cholesterol and triglycerides [3, 19, 20]. A similar beneficial effect has also been demonstrated on LDL cholesterol in type 2 diabetic subjects [21].
Furthermore, we have recently shown that long-term treatment with stevioside in combination with soy protein have a preventive effect on the development of type 2 diabetes and the metabolic syndrome in the Zucker diabetic fatty (ZDF) rat [22]. In this prevention study we found a 19 % reduction in blood glucose compared to a control group after 10 wk of treatment, as well as an improvement of the blood lipid profile [22]. The results of this study encouraged us to investigate if the combination of stevioside and soy-protein isolate, in a treatment situation, also possesses beneficial effects in the treatment of type 2 diabetes and its cardiovascular risk factors, such as hyperglycemia, hypertension, dyslipidemia and weight gain, in the diabetic GK rat.
Methods
Study design
Adult male GK rats weighing 200-230 g at the age of 20 wk were obtained from Takeda Chemical, Tokyo, Japan, and bred locally at Bomholtgaard Breeding and Research Centre, Ry, Denmark. The animals were divided into four groups (n = 12 in each group), kept in individual cages and fed for 4 wk with four different pellet diets:
Group A received a standard carbohydrate-rich laboratory diet (chow).
Group B received chow + stevioside (0.03 g per kg body weight (BW) per day).
Group C received 80% SPI (Abalon®) + 20% chow.
Group D received 80% SPI + 20% chow + stevioside (0.03 g/kg BW/day).
All diets contained similar amounts of vitamins and minerals. Fasting blood glucose, blood pressure, body weight and food intake were measured every week during the 4 wk period. At week 3 an intra-arterial glucose tolerance test was performed (see below). Water was given ad libitum and a light cycle of 12 h/12 h was used. The experiment was performed in accordance with the guidelines of the Danish Council for Animal Experiments.
Stevioside and soy protein dietary supplement
The stevioside molecule (19-O-β-glucopyranosyl-13-O-β-glucopyranosyl (1-2)-β-glucopyranosyl-steviol, Wako Chemicals, Japan) is composed of steviol, a diterpenic carboxylic alcohol, and 3 D-glucose molecules. It was dissolved in tap water and given to the GK rats in small bottles containing approximately 8 ml, dependent on the weight of the animals (0.03 g/kg BW/day). SPI was obtained from NutriPharma A/S, London, England, mixed with chow diet (20%) and made into pellets (Brogaarden, Denmark). SPI has a high isoflavone content (approximately 3%) and 40% cotyledon fiber. Isoflavones are phytoestrogens, which are normally divided into 3 major classes, namely isoflavones, lignans, and coumestans. The major bioactive isoflavones are genistein and daidzein, which are derived from the precursor biochanin A and formononetin, respectively. The amount of isoflavones in soybean varies according to the type of soybean, geographic area of cultivation and harvest year. In addition, the isoflavone content of different soy products varies substantially as a result of different processing methods.
Glucose tolerance test
After 3 wk of feeding the GK rats were anesthetized by subcutaneous injection (1 ml/kg BW) of a mixture of 0.08 mg/ml phentanyl citrate, 2.5 mg/ml phluanisone (Janssen Pharmaceutica, Beerse, Belgium) and 1.25 mg/ml midazolame (Dumex-Alpharma, Oslo, Norway). A midline incision was made in the frontal part of the neck and the right artery was isolated. The cephalic part of the artery was closed by suture and the catheter held in position by ligatures. A hole in the artery was made caudal to the closing suture and a catheter (Tygon Microbore Tubing, Norton Performance Plastics, Akron, OH; inner diameter 0.40 mm, outer diameter 0,78 mm) was inserted 30 mm into the artery and held in position by ligatures. The free end of the catheter was exteriorized at the neck leaving 2.5 cm of the catheter outside the animal. The catheter was filled with 0.9 % saline containing 10 U/nl heparin (Løvens Kemiske Fabrik, Ballerup, Denmark) and closed with a fishing line inserted into the lumen. After surgery, the animals were given 0.08 mg Naloxone intramuscularly (IM) as antidote (DuPont Pharmaceuticals, Hertfordshire, UK). Then the animals were caged individually with free access to food and water. The catheters were flushed daily with 0.2 ml saline/heparin to keep them open. After 7 days recovery, an intra-arterial glucose tolerance test (IAGTT) was performed. Food was withheld from the animals for 12 h (water ad libitum) before the experiments started at 8 am.
The animals were moving freely in separate plastic cylinders. The intra-arterial catheter was connected to a polyethylene tube system (PE20, Intramedic, Becton Dickison, Sparks, MD) allowing blood sampling and infusion. Blood samples were drawn at time points from -15 to 240 minutes. Immediately after the 0 minute sample D-glucose (2 g/kg BW dissolved in 0.9% saline) was administered as a bolus infusion over 20 seconds. Blood samples (250 μl) were drawn in chilled tubes containing a 3 μl aprotinin/heparin mix (7.7 mg/ml aprotinin and 2.300 IU/ml heparin). The samples were then centrifuged (10 min at 4°C and 4.000 rpm) and plasma was frozen for subsequent analysis of insulin, glucose and glucagon. The hemoglobin phase of the blood samples was dissolved in isotonic saline and infused back into the animal to avoid volume depletion. Subsequently, the animals continued the feeding study for 6 more days, and were then fasted overnight before a large blood sample was drawn in the morning (for analysis of total cholesterol, TG and FFA).
Measurement of tail blood pressure, body weight and food intake
Tail blood pressure (systolic) was measured using the Automatic System 209002 (TSE GmbH, Bad Homburg, Germany). Blood pressure was measured once a week, at the same time each week.
Assays
Fasting blood glucose was measured on whole blood using One Touch equipment (Lifescan, a Johnson and Johnson company, USA). Blood samples taken during the glucose tolerance test were centrifuged (10 min at 4°C and 4.000 rpm), and glucose was determined using the GLU MPR Glucose/GOD-PAP method (Boehringer Mannheim GmbH, Mannheim, Germany). Plasma insulin and glucagon were analyzed by radioimmunoassay (RIA) kits (Linco Research, St Charles, MO). The antibody in the glucagon kit is specific for pancreatic glucagon and does not cross react with other islet polypeptides. The sensitivity of the glucagon assay is 20 pg/ml. For the insulin secretion study, insulin was analyzed by RIA using a guinea pig antiporcine insulin antibody (Novo Nordisk A/S, Bagsvaerd, Denmark) and mono-125I (tyr A14)-labeled human insulin (Novo Nordisk) as tracer as well as porcine insulin standard (Novo Nordisk). Bound and free radioactivity was separated using ethanol.
Statistics
Statistical analysis was carried out using the two-way ANOVA test. Differences were considered to be being significant if p < 0.05. Student's unpaired t-test was used to compare groups (e.g. group A and D). All data are presented as mean ± SEM unless stated otherwise. The incremental area was calculated as the area under the curve above basal (IAUC). The total area under the curve (TAUC) was calculated as the area above zero. With the aim to reduce the amount of data, mean treatment difference (%) is shown for selected groups only (Table 1).
Table 1. The effect of stevioside and/or soy-protein isolate (SPI) on glycemic control and cardiovascular risk markers in the type 2 diabetic GK rat.
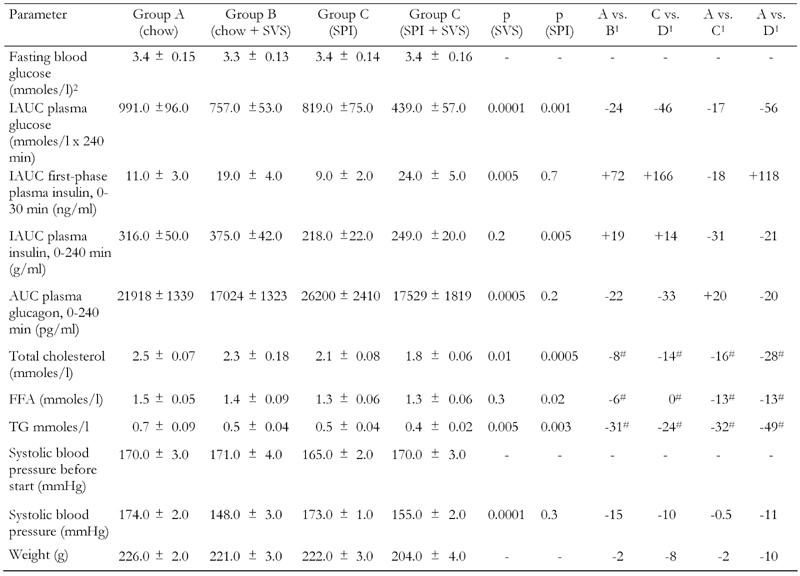
Legend: Data are expressed as mean ± SEM, unless otherwise indicated. Mean treatment difference (%). - indicates decrease, + indicates increase after 4 wk of treatment (# indicates that the animals were treated for 5 wk). 1 Mean treatment difference (%). 2 whole blood before start.
Results
Before the start of the treatment, neither control nor the treatment groups of animals exhibited any significant differences in fasting whole blood glucose (Table 1). Figure 2 (A and B) shows that plasma glucose was suppressed after intra-arterial injection of glucose (2.0 g/kg) in the stevioside-treated groups (IAUC, group A (991 ± 96 mmoles/l x 240 min) vs. group B (757 ± 53 mmoles/l x 240 min), 24% reduction, and group C (819 ± 75 mmoles/l x 240 min) vs. group D (439 ± 57 mmoles/l x 240 min), 46% reduction; p < 0.001), see Table 1. SPI, mixed with normal chow food in a 80/20% ratio, also suppressed plasma glucose (IAUC, group A (991 ± 96 mmoles/l x 240 min) vs. group C (819 ± 75 mmoles/l x 240 min) by 17%, and between group B (757 ± 53 mmoles/l x 240 min) vs. D (439 ± 57 mmoles/l x 240 min) by 42%; p < 0.001). In a comparison between groups A and D, we found a reduction in plasma glucose of 56% (IAUC, group A (991 ± 96 mmoles/l) vs. group D (439 ± 57 mmoles/l); p < 0.001). All data are shown in Table 1.
Figure 2. Intra-arterial glucose tolerance tests in type 2 diabetic GK rats after 4 wk of treatment with four different diets.
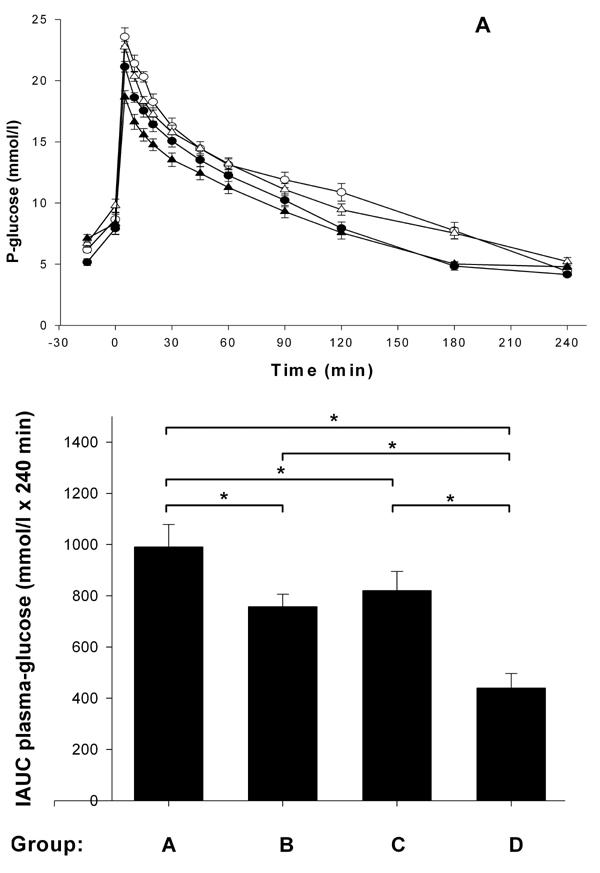
Group A (○) with standard carbohydrate-rich laboratory diet (chow), group B (●) with chow + stevioside, group C (∆) with 80% SPI + 20% chow and group D (▲) with 80% SPI + 20% chow + stevioside. Glucose was administered at time point 0 (A). B shows the IAUCglucose. Results are shown as mean ± SEM, n = 12 in each group. * p < 0.001.
Figure 3A shows that stevioside-fed animals had a significantly higher first-phase insulin response during the IAGTT than the respective control groups (IAUC, group A (11 ± 3 ng/ml x 30 min) vs. group B (19 ± 4 ng/ml x 30 min), i.e. a 72% increase in insulin, and group C (9 ± 2 ng/ml x 30 min) vs. group D (24 ± 5 ng/ml x 30 min), i.e. a 166% increase in insulin; p < 0.01) (Table 1). In the entire observation period, the insulin response did not differ significantly between the group treated with stevioside and the control groups (IAUC, group A (316 ± 50 ng/ml x 240 min) vs. group B (375 ± 42 ng/ml x 240 min) and group C (218 ± 22 ng/ml x 240 min) vs. group D (249 ± 20 ng/ml x 240 min, NS). SPI did not change first-phase insulin, but decreased insulin during the entire observation period (IAUC, group A (316 ± 50 ng/ml x 240 min) vs. group C (218 ± 22 ng/ml x 240 min), a reduction of 31%, and a 34% reduction by comparison of group B (375 ± 42 ng/ml x 240 min) with group D (249 ± 20 ng/ml x 240 min); p < 0.01). When we compared groups A and D we found an increase in first-phase insulin of 118% (IAUC, group A (11 ± 3 ng/ml x 30 min) vs. group D (24 ± 5 ng/ml x 30 min); p < 0.05). All data are shown in Table 1.
Figure 3. The effects of 4 wk of treatment with four different diets.
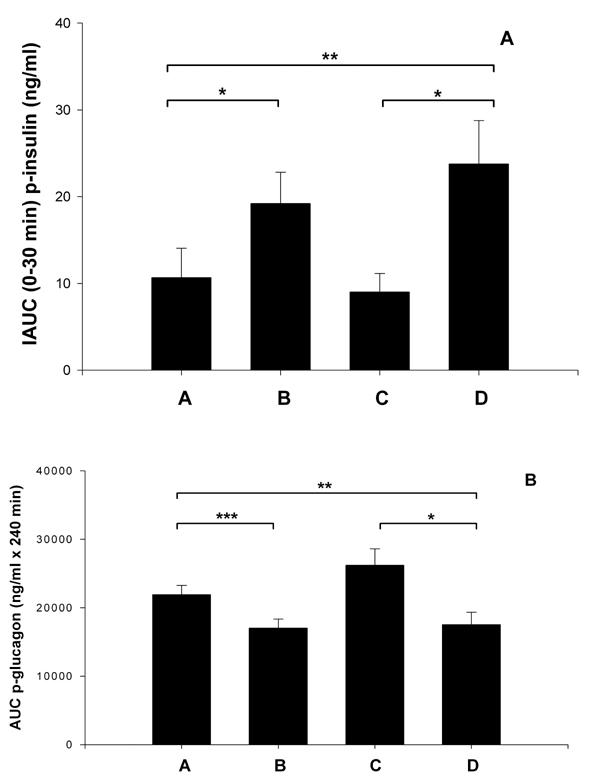
Group A with standard carbohydrate-rich laboratory diet (chow), group B with chow + stevioside (0.03 g/kg BW/day), group C with 80% SPI + 20% chow and group D with 80% SPI + 20% chow + stevioside (0.03 g/kg BW/day), on first-phase insulin (A) and glucagon (B) during IAGTT. Values are shown as mean ± SEM, n = 12 in each group. * p < 0.01, ** p < 0.05, *** p < 0.001.
Figure 3B illustrates that stevioside-treated animals had a much lower glucagon level than the control group during the IAGTT (AUC group A (21918 ± 1339 pg/ml x 240 min) vs. group B (17023 ± 1323 pg/ml x 240 min) i.e. a 22% reduction, and group C (26200 ± 2410 pg/ml x 240 min) vs. group D (17529 ± 1819 pg/ml x 240 min), i.e. a 33% reduction in glucagon; p < 0.001). SPI did not significantly change the glucagon level (group A (21918 ± 1339 pg/ml x 240 min) vs. group C (26200 ± 2410 pg/ml x 240 min) and group B (17023 ± 1323 pg/ml x 240 min) vs. group D (17529 ± 1819 pg/ml x 240 min), NS. By comparing groups A and D we found a significant change in the glucagon level (group A (21818 ± 1339 pg/ml) vs. group D (17529 ± 1819 pg/ml), p = 0.05). Data are shown in Table 1.
Figure 4A shows the effect of the four different diets on plasma cholesterol after 5 wk of treatment. Stevioside was able to lower the plasma cholesterol level after 5 wk of treatment (group A (2.5 ± 0.07 mmoles/l) vs. group B (2.3 ± 0.18 mmoles/l) by 8% and for group C (2.1 ± 0.08 mmoles/l) vs. group D (1.8 ± 0.06 mmoles/l) by 14%; p < 0.01). SPI seems to have a more pronounced effect on cholesterol levels (group A (2.5 ± 0.07 mmoles/l) vs. group C (2.1 ± 0.08 mmoles/l) i.e. a reduction by 16%, and group B (2.3 ± 0.18 mmoles/l) vs. group D (1.8 ± 0.06 mmoles/l) effectively a 21% decrease; p < 0.001). When we compared groups A and D, we found a total cholesterol reduction of 28% (group A (2.5 ± 0.07 mmoles/l) vs. group D (1.8 ± 0.06 mmoles/l); p < 0.001). Data are shown in Table 1.
Figure 4.
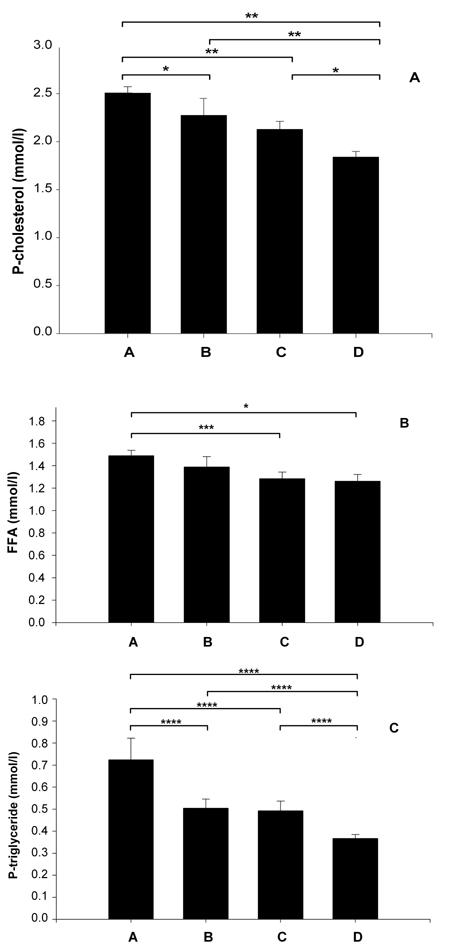
The effects on fasting plasma cholesterol (A), FFA (B) and TG (C) after 5 wk of treatment with four different diets. Group A with standard carbohydrate-rich laboratory diet (chow), group B with chow + stevioside, group C with 80% SPI + 20% chow and group D with 80% SPI + 20% chow + stevioside. Values are shown as mean ± SEM, n = 12 in each group. * p < 0.01, ** p < 0.001, *** p < 0.05, **** p < 0.005.
Figure 4B shows that only SPI, had an effect by reducing the level of FFA by 13% (group A (1.5 ± 0.05 mmoles/l) vs. group C (1.3 ± 0.06 mmoles/l); p < 0.05) and by 8% between group B (1.4 ± 0.09 mmoles/l) vs. group D (1.3 ± 0.06 mmoles/l), NS. A comparison of groups A and D shows a 13% reduction in FFA levels (group A (1.5 ± 0.05 mmoles/l) vs. group D (1.3 ± 0.06 mmoles/l); p < 0.01). Data are summarized in Table 1.
Figure 4C illustrates that TG were decreased by stevioside and SPI. Stevioside was able to reduce plasma TG by 31% (group A (0.72 ± 0.9 mmoles/l) vs. B (0.5 ± 0.04 mmoles/l)) and by 24% when comparing group C (0.49 ± 0.04 mmoles/l) vs. D (0.37 ± 0.02 mmoles/l), with p < 0.005 in both cases. As mentioned above, SPI also reduced the TG level significantly by 32% (group A (0.72 ± 0.09 mmoles/l) vs. group C (0.49 ± 0.04 mmoles/l) and by 26% when comparing group B (0.5 ± 0.04 mmoles/l) vs. group D (0.37 ± 0.02 mmoles/l), with p < 0.005 in both cases. A comparison between group A (0.72 ± 0.09 mmoles/l) and group D (0.37 ± 0.02 mmoles/l) shows a 49% decrease in TG. Data are presented in Table 1.
As illustrated in Figure 5, before the start of treatment with stevioside and SPI, there were no significant differences in systolic blood pressure between the control and the experimental groups of rats (p = 0.4) (Table 1). After the 4-wk study period, a significant, 10-15% decrease in systolic blood pressure was observed in the groups treated with stevioside (group A (174 ± 2 mmHg) vs. group B (148 ± 3 mmHg), 15% decrease and group C (173 ± 1 mmHg) vs. group D (155 ± 2 mmHg), 10% decrease; p < 0.001). Stevioside resulted in a progressive lowering of blood pressure after 2 wk of treatment and beyond (p < 0.05) (Figure 5). SPI did not change the blood pressure significantly (group A (174 ± 2 mmHg) vs. group C (173 ± 1 mmHg) and group B (148 ± 3 mmHg) vs. group D (155 ± 2 mmHg); p = 0.3. A comparison between groups A and D showed an 11% reduction in the systolic blood pressure (group A (174 ± 2 mmHg) vs. group D (155 ± 2 mmHg); p < 0.001).
Figure 5. The effects of the four different diets after 4 wk of treatment on systolic blood pressure.
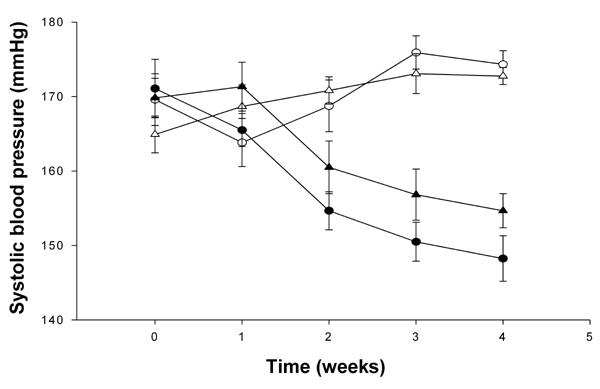
Group A (○) with standard carbohydrate-rich laboratory diet (chow), group B (●) with chow + stevioside, group C (∆) with 80% SPI + 20% chow and group D (▲) with 80% SPI + 20% chow + stevioside. Data are shown as mean ± SEM, n = 12 in each group.
Stevioside was also observed to reduce body weight. A reduction of 2% was found by comparison between group A (226 ± 2 g) and group B (221 ± 3 g) and an 8% reduction was found by comparison of group C (222 ± 3 g) and group D (204 ± 4 g), p < 0.001 (Table 1 and Figure 6). Similarly, SPI decreased the body weight by 2% (group A (226 ± 2 g) vs. group C (222 ± 3 g)) and by 8% when comparing group B (221 ± 3 g) with group D (204 ± 4 g), both with p < 0.001. Stevioside in combination with SPI had an even more significant effect on the body weight in reducing it by 10% after the 4 wk treatment period (group A (226 ± 2 g) vs. group D (204 ± 4 g); p < 0.00049) (Table 1 and Figure 6).
Figure 6. Body weight after treatment with four different diets after 4 wk.
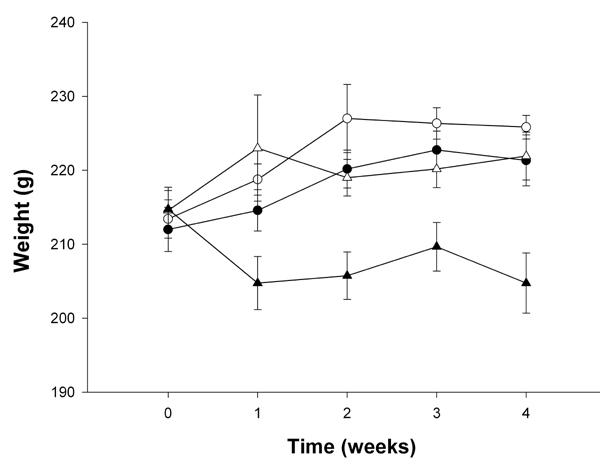
Group A (○) with standard carbohydrate-rich laboratory diet (chow), group B (●) with chow + stevioside, group C (∆) with 80% SPI + 20% chow and group D (▲) with 80% SPI + 20% chow + stevioside. Data are shown as mean ± SEM, n = 12 in each group.
Discussion
In the present study we have demonstrated that the long-term administration of stevioside in combination with SPI (Abalon®) exerts beneficial effects on blood glucose, blood pressure and lipid levels in the type 2 diabetic GK rat. Our study corroborates previous results, which demonstrated that stevioside, the main component of the dry matter of leaves of SrB, has per se an anti-hyperglycemic and blood pressure-lowering effect in the diabetic GK rat [12]. In addition to the previous results, the present study have shown that the glucose lowering and insulinotropic effects of stevioside are enhanced when given in combination with SPI. Only plasma glucose was lowered by SPI, but it did not have a significant effect on glucagon levels, as shown in the comparison between groups A and C (Figures 2 and 3). However, there are obviously differences depending on which compound is used. While rebaudioside A did not show significant effects on glucose, glucagon and insulin responses in GK rats, an earlier study demonstrated that the compound used in the present study had a much higher efficacy [13, 23].
Type 2 diabetes is a chronic metabolic disorder that results from insulin resistance and defects in first-phase insulin secretion. Also it is characterized by a relative excess in glucagons and pancreatic α-cell dysfunction [24]. The suppression of circulating glucagon concentrations reduces blood glucose levels [25]. Therefore, agents that inhibit glucagon secretion or action, are beneficial for diabetic patients.
In experimental diabetes, an abnormal α-cell function is characterized by an impaired response to glucose and certain glucose metabolites that probably results from a specific defect in glucose recognition [26]. The abnormal α-cell function seems to be attributable to insulin deficiency and also to an abnormal metabolic state secondary to relative insulin deficiency [27]. In this context, it is interesting that plasma glucagons were decreased by 29% during the IAGTT in the stevioside-treated groups compared with the control group, and that the effect seems to be further enhanced when soy protein is added, where we found a lowering effect of 49%. Similarly, stevioside has recently been shown to reduce the palmitate-stimulated glucagon release from α-cells [28]. Clearly, SPI alone had no significant effect on glucagons. Therefore, stevioside has an accentuated effect on α-cell function.
In another study, we have addressed the question whether stevioside in the fasting state causes hypoglycemia, as does the treatment with sulphonylureas [14]. In this previous study we demonstrated that a bolus infusion of stevioside in the fasting state did not cause hypoglycemia in the normal or diabetic rat. This result was further supported by an in vitro study, which shows that the effects of stevioside fade at glucose concentrations comparable with normoglycemia [11, 12]. These beneficial properties of stevioside may be of high clinical relevance for the treatment of type 2 diabetes as it seems to have the potential to become an effective and competitive therapeutic. The long-term administration of stevioside and/or soy-protein isolate in GK rats did not influence fasting blood glucose, insulin, and glucagon, while it markedly improved glucose tolerance.
The long-term prognosis of type 2 diabetes relies on the treatment of hyperglycemia, and also on coexisting conditions, such as hypertension, dyslipidemia and obesity, as delineated in the United Kingdom Prospective Diabetes Study (UKPDS) [29-32]. Consequently, the pharmacologic intervention in type 2 diabetes should also aim to lower blood pressure and lipid concentration. The present study corroborates our previous findings that long-term stevioside treatment had a significant blood pressure-lowering effect from week 2 onwards [14]. Interestingly, a blood pressure-lowering effect has also been reported in non-diabetic hypertensive subjects who were treated with stevioside for 2 years [33]. No adverse effects on lipid or glucose were reported in these non-diabetic subjects. Although the mechanism underlying the antihypertensive effect of stevioside is still not clear, it is believed that blood pressure-lowering is mediated through a calcium antagonistic mechanism [34, 35].
Many animal and human studies have examined whether the consumption of soy-containing diets has an effect on glucose and lipid metabolism. Early studies in healthy human subjects showed that soy polysaccharides reduce postprandial glucose and TG concentration [36, 37], suggesting that polysaccharides in soy may have beneficial effects against impaired glucose tolerance and hyperlipidemia. The beneficial effect may also be due to the proteins included in soy. In one study, soy protein performed better than casein by inducing a lower postprandial insulin-glucagon ratio in healthy and hypercholesterolemic subjects [38]. Soy proteins are rich in arginine and glycine, which can influence insulin and glucagon secretion from the pancreas. In this study, we observed that soy protein decreased plasma insulin compared to a normal chow diet (Table 1). Thus, the decrease in cholesterol seen with soy protein may be due to the decreased insulin-glucagon ratio caused by arginine and glycine [39]. In healthy subjects, Lang et al. observed no effect of protein supplement (soy, other vegetable protein and animal proteins) on plasma glucose, insulin, or glucagon, but the kinetics of glucose, insulin and glucagon were different after ingestion of different sources of protein in a mixed meal [40].
Recently, Lavigne et al. evaluated the effect of controlled feeding with various types of dietary proteins on glucose tolerance and insulin sensitivity in healthy Wistar rats [41]. The rats were fed isoenergetic diets containing casein, cod or soy protein for 28 days. Rats fed with cod and soy protein had lower fasting plasma glucose and insulin concentrations than rats fed with casein. After an intravenous glucose load (1.5 ml/kg body weight of 85% glucose in saline), rats fed with cod and soy protein had lower incremental areas under the glucose response curves for glucose than rats fed with casein, suggesting that cod and soy proteins improve glucose tolerance. This phenomenon is consistent with our data as IAUCglucose was decreased by 21% in the group fed with soy protein compared with normal chow diet (Table 1). This points to an improvement in peripheral insulin sensitivity. However, the lower plasma insulin concentration observed in animals fed with soy protein may be in part due to the decreased pancreatic insulin release or to the increased hepatic insulin removal, or possibly due to both in combination. Interestingly, there seems to be a markedly improvement in glucose tolerance in the group receiving both soy protein and stevioside (Table 1).
A better option would be the prevention or delay of type 2 diabetes onset and the metabolic syndrome could be prevented or delayed. In another study, we have shown that stevioside in combination with a soy-based diet was able to delay the onset of diabetes in ZDF rats if the animals were treated from the age of 8 wk onwards [22]. In this study, after 9 wk of treatment, we detected a significantly greater reduction in the IAUCglucose during the first 30 minutes of IAGTT in the stevioside-treated rather than in the non-treated group (by 19%, p < 0.001) [22].
In the present study, we detected a beneficial effect of SPI on blood lipids in the GK rat. When comparing soy protein with the chow diet, we found that SPI was able to decrease total cholesterol by 19%, FFA by 15% and TG by 47%. A synergistic effect on total cholesterol and TG was observed when stevioside was added (Table 1). We are aware that the concentration of SPI in the diet was high (80%) and that energy intake may be different because of the low fat content in the soy-based diet compared to the chow diet. However, there was no significant difference in food intake between the four groups (data not shown). Therefore, it appears that soy-based diets may provide benefits in conditions associated with impaired glucose tolerance, dyslipidemia, and reduced insulin sensitivity.
High-fiber diets are also known to have beneficial effects on the lipid metabolism. However, in the present study, it was not clear whether the beneficial effect on plasma lipids was due to soy protein, isoflavones, or cotyledon fiber. Vedavanam et al. suggested that soy isoflavones may be beneficial for diabetic subjects due to their estrogenic activity and their ability to prevent glucose-induced lipid peroxidation and because they inhibit intestinal glucose uptake by decreasing sodium-dependent glucose transporter, resulting in a reduction of postprandial hyperglycemia [42]. Mezei et al. demonstrated that isoflavones improve lipid and glucose metabolism by acting as a PPAR agonist [43]. An isoflavone-containing soy extract doubled PPAR-directed gene expression, and a similar induction was observed when the soy isoflavones genistein or daidzein were used to treat cells. This may be an explanation why isoflavones in soy protein have a beneficial effect on lipid and glucose metabolism, as also illustrated in the present study.
In conclusion, long-term treatment with stevioside in combination with SPI improves first-phase insulin response, suppresses glucagon level and has anti-hyperglycemic effects in the diabetic GK rat. The combination of stevioside and SPI seems to lower blood lipids, such as total cholesterol, TG and FFA, and elicits a body weight reduction. Additionally, stevioside causes a pronounced suppression of systolic blood pressure in the diabetic GK rat. The combination of stevioside and the SPI appears to have the potential for an effective treatment for a number of the metabolic syndrome features, such as hyperglycemia, hypertension and dyslipidemia. Whether these promising results can contribute to a new treatment or a means of prevention remains to be determined in a long-term clinical study with type 2 diabetic subjects.
Acknowledgments
The authors wish to thank Kirsten Eriksen, Dorthe Rasmussen and Tove Skrumsager for their skilful technical assistance. The study was supported by grants from the Danish Research Agency - Ministry of Science Technology and Innovation, Novo Nordisk Foundation, the Research Foundation of Aarhus University, the Faculty of Health Science (Aarhus University), Aarhus Sygehus Forskningsfond, the VELUX Foundation and Nutri Pharma ASA, Oslo, Norway, which also provided the SPI Abalon® used in this study.
References
- 1.Zimmet PJ. The global epidemiology of non-insulin dependent diabetes mellitus and the metabolic syndrome. Diabetes Complications. 1997;11:60–68. doi: 10.1016/s1056-8727(96)00090-6. [DOI] [PubMed] [Google Scholar]
- 2.Adams M, Golden D, Anthony M, Register T, William JK. The inhibitory effect of soy protein isolate on atherosclerosis in mice does not require the presence of LDL receptor or alteration of plasma lipoproteins. J Nutr. 2002;132:43–49. doi: 10.1093/jn/132.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson TB. Soy phytoestrogenes and cardiovascular disease. J Nutr. 2002;132:566S–569S. doi: 10.1093/jn/132.3.566S. [DOI] [PubMed] [Google Scholar]
- 4.Kahn SE. The relative contribution of insulin resistance and beta cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 5.Watt J, Wood C. R Soc Med; London: 1998. Talking health: conventional and complementaty approaches. [Google Scholar]
- 6.WHO Expert Committee. Technical Report Series 646. WHO; Geneva: 1980. Diabetes mellitus. [PubMed] [Google Scholar]
- 7.Soejarto DD, Kinghorn AD, Farnsworth NR. Potential sweetining agent of plant origin III: Organo leptic evaluation of Stevia leaf herbarium samples for sweetness. Econ Bot. 1983 doi: 10.1021/np50023a013. [DOI] [PubMed] [Google Scholar]
- 8.Bertoni MS. El Caa-e-he (Eupatorium rebaudianum, especies novas) Rev Agr Assuncion. 1899;1:35–37. [Google Scholar]
- 9.Oviedo CA, Fronciani G, Moreno R, Maas LC. Accion hipoglicemiante de la Stevia rebaudiana Bertoni. Excerpta Medica. 1979;209:92. [Google Scholar]
- 10.Curi R, Alvarez M, Bazotte RB, Botion LM, Godoy JL, Bracht A. Effect of Stevia rebaudiana on glucose tolerance in normal adult humans. Braz J Biol Res. 1986;19:771–774. [PubMed] [Google Scholar]
- 11.Jeppesen PB, Gregersen S, Hermansen K. Stevioside and steviol stimulate insulin secretion from isolated mouse islets. Diabetologia. 1996;A125:472. [Google Scholar]
- 12.Jeppesen PB, Gregersen S, Poulsen CR, Hermansen K. Stevioside acts directly on pancreatic beta cells to secrete insulin: Actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K-channel activity. Metabolism. 2000;49:208–214. doi: 10.1016/s0026-0495(00)91325-8. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen PB, Gregersen S, Alstrup KK, Hermansen K. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic effects in vivo: studies in the diabetic Goto-Kakizaki (GK) rats. Phytomedicine. 2002;9(1):9–14. doi: 10.1078/0944-7113-00081. [DOI] [PubMed] [Google Scholar]
- 14.Jeppesen PB, Gregersen S, Rolfsen SE, Jepsen M, Colombo M, Agger A, Xiao J, Kruhoffer M, Orntoft T, Hermansen K. Anti-hyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki (GK) rat. Metabolism. 2003;52(3):372–378. doi: 10.1053/meta.2003.50058. [DOI] [PubMed] [Google Scholar]
- 15.Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Acute effects of the diterpene glucoside, stevioside, in type 2 Diabetic patients. Metabolism. 2003;53:73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Yamakoshi J, Piskula M, Izumi T, Tobe K, Saito M, Kataoka S, Obata A, Kikuchi M. Isoflavone aglycone-rich extract without soy protein attenuates atheroscierosis development in cholesterol-fed rabbits. J Nutr. 2000;130:1887–1893. doi: 10.1093/jn/130.8.1887. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal MJ, Yaegashi S, Ahsan R, Lightfoot DA, Banz W. Differentially abundant mRNA's in rat liver in response to diets containing soy protein isolate. J Physiol Genomics. 2002;11:219–226. doi: 10.1152/physiolgenomics.00078.2002. [DOI] [PubMed] [Google Scholar]
- 18.Anthony MS. Soy and cardiovascular disease: cholesterol lowering and beyond. J Nutr. 2000;130:662S–663S. doi: 10.1093/jn/130.3.662S. [DOI] [PubMed] [Google Scholar]
- 19.Nestel P. Role of soy protein in cholesterol-lowering: hoe good is it? Arterioscler Thromb Vasc Biol. 2002;22:1743–1744. doi: 10.1161/01.atv.0000035520.25551.97. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. New Engl J Med. 1995;333:276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 21.Hermansen K, Sondergaard M, Hoje L. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care. 2001;24(2):228–233. doi: 10.2337/diacare.24.2.228. [DOI] [PubMed] [Google Scholar]
- 22.Dyrskog SE, Jeppesen PB, Colombo M, Abudula R, Hermansen K. Preventive effects of soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in Zucker Diabetic fatty rats. Metabolism. 2005;54:1181–1188. doi: 10.1016/j.metabol.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Dyrskog SE, Jeppesen PB, Chen J, Christensen LP, Hermansen K. The diterpene glycoside, rebaudioside A, does not improve glycemic control or affect blood pressure after eight weeks treatment in the Goto-Kakizaki rat. Rev Diabet Stud. 2005;2(2):84–91. doi: 10.1900/RDS.2005.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unger RH. Role of glucagon in the pathogenesis of diabetes: The status of the controversy. Metabolism. 1978;27:1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 25.Shah P, Vella A, Basu A. Lack of suppression of glucagon contributes to post-prandial hyperglycaemia in subject with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–4059. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 26.Hermansen K. Characterisation of the abnormal pancreatic D and A cell function in streptozotocin diabetic dogs: studies with D-glyceraldehyde, dihydroxyacetone, D-mannoheptulose, D-glucose and L-arginine. Diabetologia. 1981;21:489–494. doi: 10.1007/BF00257791. [DOI] [PubMed] [Google Scholar]
- 27.Hermansen K, Schmitz O, Orskov H. Reversal of D- and A-cell insensitivity to glucose in alloxan-diabetic dogs by treatment with the artificial beta cell (Biostator) Diabetes. 1985;34:260–266. doi: 10.2337/diab.34.3.260. [DOI] [PubMed] [Google Scholar]
- 28.Hong J, Chen L, Jeppesen PB, Nordentoft I, Hermansen K. Stevioside counteract the alpha cell hypersecretion caused by long-term palmitate exposure. Am J Physiol Endocrinol Metab. 2006;290:416–422. doi: 10.1152/ajpendo.00331.2005. [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type II diabetes (UKPDS33) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 30.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complication in type II diabetes (UKPDS 38) BMJ. 1998;318:703–713. [PMC free article] [PubMed] [Google Scholar]
- 31.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type II diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alder AI, Stratton IM, Niel HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complication of type II diabetes (UKPDS): Prospective observational study. BMJ. 2000;321(7258):412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh MH, Chan P, Sue YM, Liu JC, Liang TH, Huang TY, Tomlinson B, Chow MS, Fao PF, Chen Y. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension: a two-year, randomized, placebo-controlled study. J Clin Ther. 2003;25:2797–2808. doi: 10.1016/s0149-2918(03)80334-x. [DOI] [PubMed] [Google Scholar]
- 34.Melis MS, Sainati AR. Effect of calcium and verapamil on renal function of rats during treatment with Stevioside. J Ethnopharmacol. 1991;33:257–262. doi: 10.1016/0378-8741(91)90086-s. [DOI] [PubMed] [Google Scholar]
- 35.Melis MS. Influence of calcium on the blood pressure and renal effects of stevioside braz. J Med Biol Res. 1992;25:943–949. [PubMed] [Google Scholar]
- 36.Tsai AC, Mott EL, Owen GM, Bennick MR, Lo GS, Steinke FH. Effects of soy polysaccharide on gastrointestinal function, nutrient balance, steroid excretions, glucose tolerance, serum lipids, and other parameters in humans. Am J Clin Nutr. 1983;38(4):504–511. doi: 10.1093/ajcn/38.4.504. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed M, Gannon MC, Nuttall FQ. Postprandial plasma glucose, insulin, glucagon and triglyceride responses to a standard diet in normal subjects. Diabetologia. 1976;12:61–67. doi: 10.1007/BF01221966. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez A, Hubbard RW. Plasma amino acids and the insulin/glucagon ratio as an explanation for the dietary protein modulation of atherosclerosis. Med Hypoth. 1991;36:27–32. doi: 10.1016/0306-9877(91)90160-z. [DOI] [PubMed] [Google Scholar]
- 39.Howe JC. Postprandial response of calcium metabolism in postmenopausal women to meals varying in protein level/source. Metabolism. 1990;39:1246–1252. doi: 10.1016/0026-0495(90)90178-f. [DOI] [PubMed] [Google Scholar]
- 40.Lang V, Bellisle F, Oppert JM, Craplet C, Bornet FR, Slama G, Guy-Grand B. Satiating effect of protein in healthy subejcts: a comparison of egg albumin, casein, gelatin, pea protein, and wheat gluten. Am J Clin Nutr. 1998;67(6):1197–1204. doi: 10.1093/ajcn/67.6.1197. [DOI] [PubMed] [Google Scholar]
- 41.Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab. 2000;278(3):E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- 42.Vedavanam K, Srijayanta S, O'Reilly J, Raman A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE) Phytother Res. 1999;13(7):601–608. doi: 10.1002/(sici)1099-1573(199911)13:7<601::aid-ptr550>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133(5):1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]


