Abstract
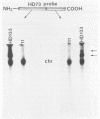
Bacillus thuringiensis subsp. aizawai HD133 is one of several strains particularly effective against Plodia interpunctella selected for resistance to B. thuringiensis subsp. kurstaki HD1 (Dipel). B. thuringiensis subsp. aizawai HD133 produces inclusions containing three protoxins, CryIA(b), CryIC, and CryID, and the CryIC protoxin has been shown to be active on resistant P. interpunctella as well as on Spodoptera larvae. The CryIA(b) protoxin is very similar to the major one in B. thuringiensis subsp. kurstaki HD1, and as expected, this protoxin was inactive on resistant P. interpunctella. A derivative of B. thuringiensis subsp. aizawai HD133 which had been cured of a 68-kb plasmid containing the cryIA(b) gene produced inclusions comprising only the CryIC and CryID protoxins. Surprisingly, these inclusions were much less toxic for resistant P. interpunctella and two other Lepidoptera than those produced by the parental strain, whereas the soluble protoxins from these strains were equally effective. In contrast, inclusions from the two strains were about as active as soluble protoxins for Spodoptera frugiperda larvae, so toxicity differences between inclusions may be due to the solubilizing conditions within particular larval guts. Consistent with this hypothesis, it was found that a higher pH was required to solubilize protoxins from inclusions from the plasmid-cured strain than from B. thuringiensis subsp. aizawai HD133, a difference which is probably attributable to the absence of the CryIA(b) protoxin in the former. The interactions of structurally related protoxins within an inclusion are probably important for solubility and are thus another factor in the effectiveness of B. thuringiensis isolates for particular insect larvae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Beckman W., Dunn P. Bacillus thuringiensis and related insect pathogens. Microbiol Rev. 1986 Mar;50(1):1–24. doi: 10.1128/mr.50.1.1-24.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Beckman W. Transfer of chromosomal genes and plasmids in Bacillus thuringiensis. Appl Environ Microbiol. 1987 Jul;53(7):1525–1530. doi: 10.1128/aem.53.7.1525-1530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson H., Dunn P. E., Strnad S., Aronson A. I. Specificity of Bacillus thuringiensis for lepidopteran larvae: factors involved in vivo and in the structure of a purified protoxin. Mol Microbiol. 1989 Nov;3(11):1533–1543. doi: 10.1111/j.1365-2958.1989.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Bietlot H. P., Vishnubhatla I., Carey P. R., Pozsgay M., Kaplan H. Characterization of the cysteine residues and disulphide linkages in the protein crystal of Bacillus thuringiensis. Biochem J. 1990 Apr 15;267(2):309–315. doi: 10.1042/bj2670309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastidar P. G., Nickerson K. W. Interchain crosslinks in the entomocidal Bacillus thuringiensis protein crystal. FEBS Lett. 1979 Dec 15;108(2):411–414. doi: 10.1016/0014-5793(79)80575-x. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Geiser M., Schweitzer S., Grimm C. The hypervariable region in the genes coding for entomopathogenic crystal proteins of Bacillus thuringiensis: nucleotide sequence of the kurhd1 gene of subsp. kurstaki HD1. Gene. 1986;48(1):109–118. doi: 10.1016/0378-1119(86)90357-4. [DOI] [PubMed] [Google Scholar]
- Haider M. Z., Ward E. S., Ellar D. J. Cloning and heterologous expression of an insecticidal delta-endotoxin gene from Bacillus thuringiensis var. aizawai IC1 toxic to both lepidoptera and diptera. Gene. 1987;52(2-3):285–290. doi: 10.1016/0378-1119(87)90055-2. [DOI] [PubMed] [Google Scholar]
- Haima P., Bron S., Venema G. The effect of restriction on shotgun cloning and plasmid stability in Bacillus subtilis Marburg. Mol Gen Genet. 1987 Sep;209(2):335–342. doi: 10.1007/BF00329663. [DOI] [PubMed] [Google Scholar]
- Hofmann C., Vanderbruggen H., Höfte H., Van Rie J., Jansens S., Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins is correlated with the presence of high-affinity binding sites in the brush border membrane of target insect midguts. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7844–7848. doi: 10.1073/pnas.85.21.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honée G., van der Salm T., Visser B. Nucleotide sequence of crystal protein gene isolated from B. thuringiensis subspecies entomocidus 60.5 coding for a toxin highly active against Spodoptera species. Nucleic Acids Res. 1988 Jul 11;16(13):6240–6240. doi: 10.1093/nar/16.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Soetaert P., Jansens S., Peferoen M. Nucleotide sequence and deduced amino acid sequence of new Lepidoptera-specific crystal protein gene from Bacillus thuringiensis. Nucleic Acids Res. 1990 Sep 25;18(18):5545–5545. doi: 10.1093/nar/18.18.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet F., Hütter R., Lüthy P. Specificity of Bacillus thuringiensis Delta-Endotoxin. Appl Environ Microbiol. 1987 Mar;53(3):500–504. doi: 10.1128/aem.53.3.500-504.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Schnepf H. E., Whiteley H. R. Diversity of locations for Bacillus thuringiensis crystal protein genes. J Bacteriol. 1983 Apr;154(1):419–428. doi: 10.1128/jb.154.1.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Whiteley H. R. Three classes of homologous Bacillus thuringiensis crystal-protein genes. Gene. 1986;43(1-2):29–40. doi: 10.1016/0378-1119(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Masson L., Préfontaine G., Péloquin L., Lau P. C., Brousseau R. Comparative analysis of the individual protoxin components in P1 crystals of Bacillus thuringiensis subsp. kurstaki isolates NRD-12 and HD-1. Biochem J. 1990 Jul 15;269(2):507–512. doi: 10.1042/bj2690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughey W. H. Insect Resistance to the Biological Insecticide Bacillus thuringiensis. Science. 1985 Jul 12;229(4709):193–195. doi: 10.1126/science.229.4709.193. [DOI] [PubMed] [Google Scholar]
- Minnich S. A., Aronson A. I. Regulation of protoxin synthesis in Bacillus thuringiensis. J Bacteriol. 1984 May;158(2):447–454. doi: 10.1128/jb.158.2.447-454.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda K., Oshie K., Shimizu M., Nakamura K., Yamamoto H., Nakayama I., Ohkawa H. Nucleotide sequence of the insecticidal protein gene of Bacillus thuringiensis strain aizawai IPL7 and its high-level expression in Escherichia coli. Gene. 1987;53(1):113–119. doi: 10.1016/0378-1119(87)90098-9. [DOI] [PubMed] [Google Scholar]
- Ostroff G. R., Pène J. J. Molecular cloning with bifunctional plasmid vectors in Bacillus subtilis. I. Construction and analysis of B. subtilis clone banks in Escherichia coli. Mol Gen Genet. 1984;193(2):299–305. doi: 10.1007/BF00330684. [DOI] [PubMed] [Google Scholar]
- Sanchis V., Lereclus D., Menou G., Chaufaux J., Guo S., Lecadet M. M. Nucleotide sequence and analysis of the N-terminal coding region of the Spodoptera-active delta-endotoxin gene of Bacillus thuringiensis aizawai 7.29. Mol Microbiol. 1989 Feb;3(2):229–238. doi: 10.1111/j.1365-2958.1989.tb01812.x. [DOI] [PubMed] [Google Scholar]
- Sanchis V., Lereclus D., Menou G., Chaufaux J., Lecadet M. M. Multiplicity of delta-endotoxin genes with different insecticidal specificities in Bacillus thuringiensis aizawai 7.29. Mol Microbiol. 1988 May;2(3):393–404. doi: 10.1111/j.1365-2958.1988.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie J., McGaughey W. H., Johnson D. E., Barnett B. D., Van Mellaert H. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science. 1990 Jan 5;247(4938):72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Iizuka T. Two types of entomocidal toxins in the parasporal crystals of Bacillus thuringiensis kurstaki. Arch Biochem Biophys. 1983 Nov;227(1):233–241. doi: 10.1016/0003-9861(83)90366-1. [DOI] [PubMed] [Google Scholar]